| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
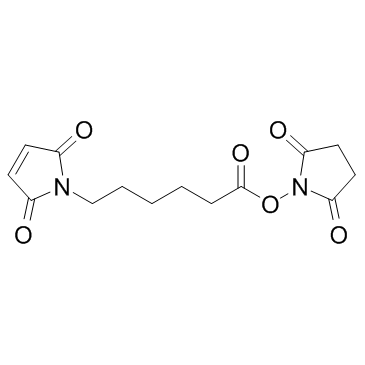 |
6-(马来酰亚胺基)己酸琥珀酰亚胺酯
CAS:55750-63-5 |
|
 |
4-(N-马来酰亚胺基甲基)环己烷-1-羧酸-3-硫代-N-琥珀酰亚胺酯钠盐
CAS:92921-24-9 |