Some in vitro receptor binding properties of [3H]eticlopride, a novel substituted benzamide, selective for dopamine-D2 receptors in the rat brain.
H Hall, C Köhler, L Gawell
文献索引:Eur. J. Pharmacol. 111 , 191-199, (1985)
全文:HTML全文
摘要
The substituted benzamide compound eticlopride, (S)-(-)-5-chloro-3-ethyl-N-[(1-ethyl-2-pyrrolidinyl) methyl]-6-methoxysalicylamide hydrochloride (FLB 131), has been shown to selectively block dopamine-D2 binding sites in the rat brain. The compound was tritium-labelled to high specific radioactivity and was used for in vitro receptor binding studies. [3H]Eticlopride was found to bind specifically to rat brain homogenates with the highest binding in the striatum and lowest in the hippocampus. The binding was saturable with a high number of binding sites (49.5 pmol/g) and with very high affinity (0.17 nM). As with other benzamides, the binding of [3H]eticlopride was highly sodium-dependent. Lesioning of the striatal neurons with ibotenic acid reduced the binding by 50% while lesioning of the nigrostriatal pathways with 6-hydroxydopamine was without effect on the observed binding. The binding of [3H]eticlopride was inhibited potently by neuroleptic drugs, while compounds known not to interact with the dopamine-D2 binding sites were inactive. It is concluded that this new dopamine-D2 antagonist may be a useful tool for the study of dopamine-D2 binding sites due to its high affinity and good selectivity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
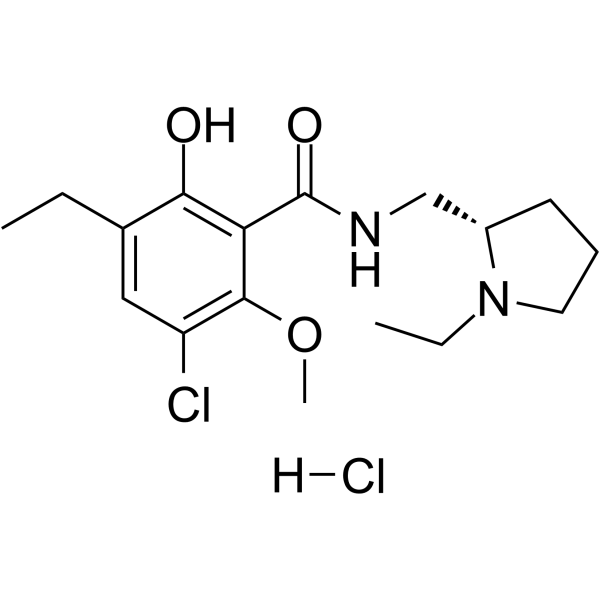 |
S-()-Eticlopride 盐酸盐
CAS:97612-24-3 |
C17H26Cl2N2O3 |
|
Dopamine D2 receptor activity modulates Akt signaling and al...
2011-04-06 [J. Neurosci. 31 , 5512-25, (2011)] |
|
Dopamine D2 receptors are involved in the regulation of Fyn ...
2016-04-01 [J. Neurosci. Res. 94 , 329-38, (2016)] |
|
Regional in vivo binding of the substituted benzamide [3H]et...
1986-01-21 [Eur. J. Pharmacol. 120 , 217-226, (1986)] |
|
Dopamine regulates angiogenesis in normal dermal wound tissu...
2011-01-01 [PLoS ONE 6(9) , e25215, (2011)] |