Nuclear pore disassembly from endoplasmic reticulum membranes promotes Ca2+ signalling competency.
Michael J Boulware, Jonathan S Marchant
文献索引:J. Physiol. 586(Pt 12) , 2873-88, (2008)
全文:HTML全文
摘要
The functionality of the endoplasmic reticulum (ER) as a Ca(2+) storage organelle is supported by families of Ca(2+) pumps, buffers and channels that regulate Ca(2+) fluxes between the ER lumen and cytosol. Although many studies have identified heterogeneities in Ca(2+) fluxes throughout the ER, the question of how differential functionality of Ca(2+) channels is regulated within proximal regions of the same organelle is unresolved. Here, we studied the in vivo dynamics of an ER subdomain known as annulate lamellae (AL), a cytoplasmic nucleoporin-containing organelle widely used in vitro to study the mechanics of nuclear envelope breakdown. We show that nuclear pore complexes (NPCs) within AL suppress local Ca(2+) signalling activity, an inhibitory influence relieved by heterogeneous dissociation of nucleoporins to yield NPC-denuded ER domains competent at Ca(2+) signalling. Consequently, we propose a novel generalized role for AL - reversible attenuation of resident protein activity - such that regulated AL (dis)assembly via a kinase/phosphatase cycle allows cells to support rapid gain/loss-of-function transitions in cellular physiology.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
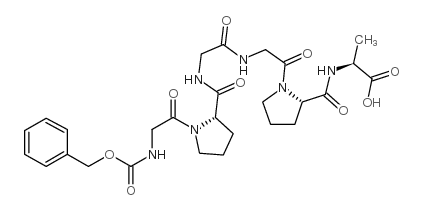 |
胶质
CAS:13075-38-2 |
C27H36N6O9 |
|
Impaired embryonic haematopoiesis yet normal arterial develo...
2008-07-09 [EMBO J. 27(13) , 1886-95, (2008)] |
|
Collagenolytic enzyme activity in human ovary: an ovulatory ...
1981-12-01 [Fertil. Steril. 36(6) , 746-50, (1981)] |
|
Cellular stretch increases superoxide production in the thic...
2008-02-01 [Hypertension 51(2) , 488-93, (2008)] |
|
Rab4 facilitates cyclic adenosine monophosphate-stimulated b...
2008-11-01 [Hepatology 48(5) , 1665-70, (2008)] |
|
Liver is able to activate naïve CD8+ T cells with dysfunctio...
2009-01-01 [PLoS ONE 4(10) , e7619, (2009)] |