Chiral building blocks: enantioselective syntheses of benzyloxymethyl phenyl propionic acids.
Jack G Parsons, Danuta Stachurska-Buczek, Neil Choi, Peter G Griffiths, Daniel A Huggins, Beata M Krywult, Sharon T Marino, Thao Nguyen, Craig S Sheehan, Ian W James, Andrew M Bray, Jonathan M White, Rustum S Boyce
文献索引:Molecules 9(6) , 449-58, (2004)
全文:HTML全文
摘要
The synthesis of (2S)-2-benzyloxymethyl-3-(2-fluoro-4-methoxyphenyl)- propionic acid, (2S)-2-benzyloxymethyl-3-(2-fluoro-4-methylphenyl)propionic acid and (2S)-2-benzyl-oxymethyl-3-(2,4-dimethylphenyl)propionic acid has been achieved by TiCl4 mediated alkylation of the corresponding (4R)-4-benzyl-3-[3-(2-fluoro-4-methoxyphenyl-, 2-fluoro-4-methylphenyl-, 2,4- dimethylphenyl-)propionyl]-2-oxazolidinones, followed by hydrolysis of the chiral auxiliary. The stereochemistry of the alkylation reaction was confirmed by an X-ray crystal structure of (4R)-4-benzyl-3-[(2S)-2-benzyloxymethyl-3-(2- fluoro-4-methylphenyl)propionyl]-2-oxazolidinone.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
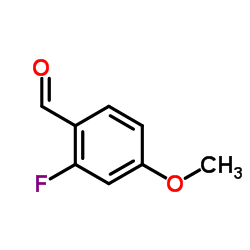 |
2-氟-4-甲氧基苯甲醛
CAS:331-64-6 |
C8H7FO2 |
|
Synthesis, cytotoxic activity and docking studies of new 4-a...
[Med. Chem. Res. 24(8) , 3305-3313, (2015)] |
|
Tyrosine Hydroxylase Inhibitors. Synthesis and Activity of S...
[J. Med. Chem. 10(6) , 1008-1014, (1967)] |
|
Indium trifluoride: A highly efficient catalyst for the synt...
[J. Fluor. Chem. 142 , 45-51, (2012)] |
|
Fluorinated derivatives of titanocene Y: synthesis and cytot...
[European J. Org. Chem. 26 , 4074-4082, (2008)] |
|
Polystyrene-Supported p-Toluenesulfonic Acid: A New, Highly ...
[Synlett 23(20) , 2985-2991, (2012)] |