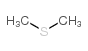
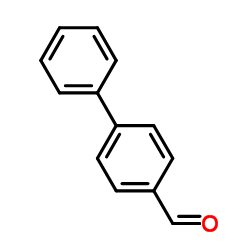
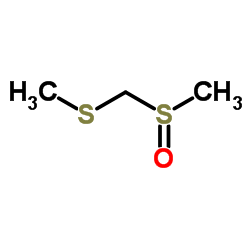
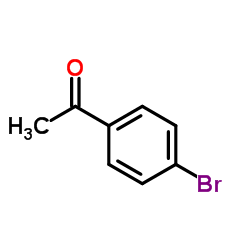
|
~94% |
|
~82% |
|
~% |
|
~% |
|
~16% |
|
~88% |
|
~93% |
|
~87% |
|
~91% |
|
~93% |
|
~87% |
|
~62% |
|
~82% |
|
~% |
|
~88% |
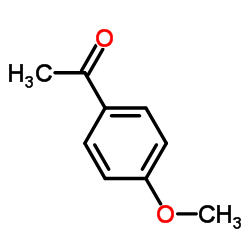
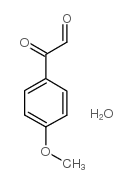

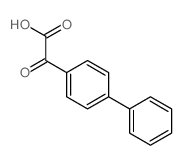
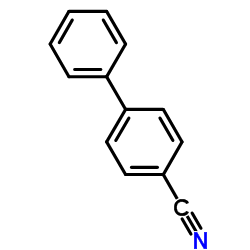
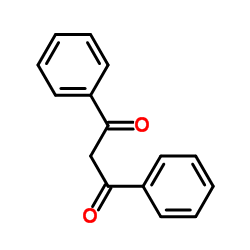
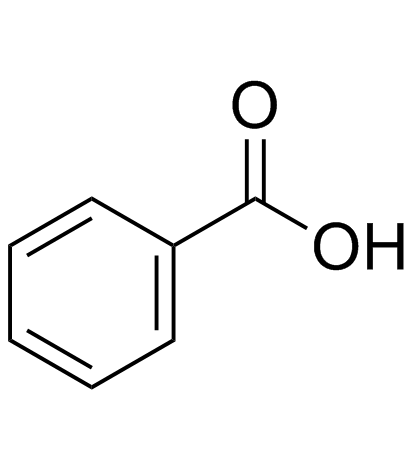
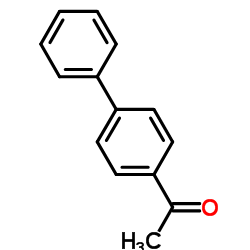
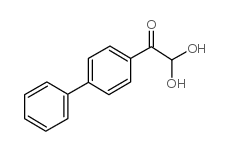

![Sulfonium, (2-[1,1'-biphenyl]-4-yl-2-oxoethyl)dimethyl-, bromide结构式](https://image.chemsrc.com/caspic/236/5697-41-6.png)

![1-([1,1'-biphenyl]-4-yl)-2-(methylthio)ethan-1-one结构式](https://image.chemsrc.com/caspic/145/46817-48-5.png)