| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
N,N',N''-三乙酰基壳三糖
CAS:38864-21-0 |
|
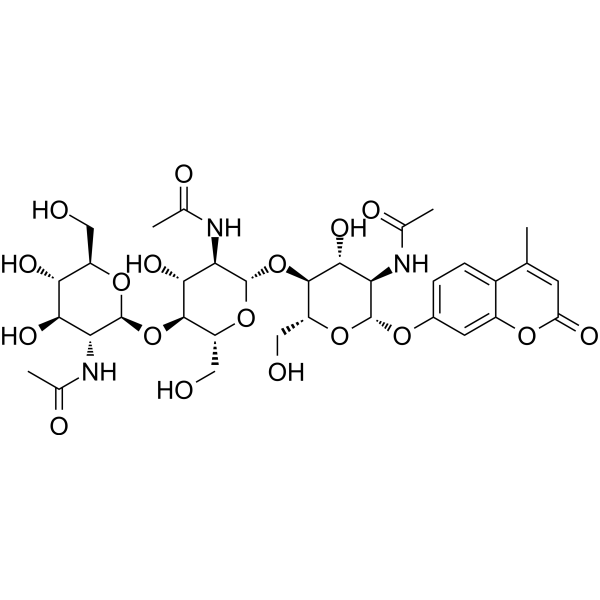 |
4-甲基伞形酮基Β-D-N,N`,N``-三乙酰基壳三糖苷
CAS:53643-13-3 |