Crystal structure of bifunctional 5,10-methylenetetrahydrofolate dehydrogenase/cyclohydrolase from Thermoplasma acidophilum.
Won Ho Lee, Min Woo Sung, Jae Hee Kim, Young Kwan Kim, Arum Han, Kwang Yeon Hwang
文献索引:Biochem. Biophys. Res. Commun. 406(3) , 459-63, (2011)
全文:HTML全文
摘要
Folate co-enzymes play a pivotal role in one-carbon transfer cellular processes. Many eukaryotes encode the tri-functional tetrahydrofolate dehydrogenase/cyclohydrolase/synthetase (deh/cyc/syn) enzyme, which consists of a N-terminal bifunctional domain (deh/cyc) and a C-terminal monofunctional domain (syn). Here, we report the first analogous archeal enzyme structures, for the bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase from Thermoplasma acidophilum (TaMTHFDC) as the native protein and also as its NADP complex. The TaMTHFDC structure is a dimer with a polar interface, as well as a NADP binding site that shows minor conformational change. The orientations of the residues in the NADP binding site do not change on ligand binding, incorporating three water molecules which are hydrogen bonded with phosphate groups of NADP in the structure of the complex. Our structural information will contribute to an improved understanding of the basis of THF and one-carbon metabolism.Copyright © 2011 Elsevier Inc. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
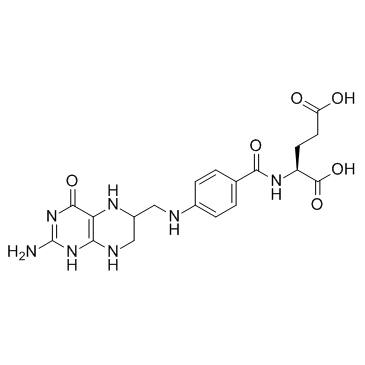 |
四氢叶酸
CAS:135-16-0 |
C19H23N7O6 |
|
Quantitation of 5-Methyltetrahydrofolic Acid in Dried Blood ...
2015-01-01 [PLoS ONE 10 , e0143639, (2015)] |
|
Arg-265: a critical residue of L.donovani cytosolic SHMT in ...
2015-02-01 [Exp. Parasitol. 149 , 16-23, (2015)] |
|
Folate levels measured by LC-MS/MS in patients with colorect...
2014-12-01 [Cancer Chemother. Pharmacol. 74(6) , 1167-74, (2014)] |
|
Folate in demethylation: the crystal structure of the rat di...
2014-07-11 [Biochem. Biophys. Res. Commun. 449(4) , 392-8, (2014)] |
|
A pharmacokinetic and pharmacodynamic investigation of Moduf...
2015-01-01 [Cancer Chemother. Pharmacol. 75(1) , 37-47, (2015)] |