Synthesis and structural characterization of oaklin-catechins.
André Sousa, Ana Fernandes, Nuno Mateus, Victor De Freitas
文献索引:J. Agric. Food Chem. 60(6) , 1528-34, (2012)
全文:HTML全文
摘要
Condensation reactions of procyanidin dimer B4 with two representative oak wood cinnamic aldehydes (coniferaldehyde and sinapaldehyde) were conducted in winelike model solutions. Coniferaldehyde led to the formation of guaiacylcatechin-pyrylium-catechin (GCP-catechin, 737 m/z), whereas sinapaldehyde led to the formation of syringylcatechin-pyrylium-catechin (SCP-catechin, 767 m/z). The former was also structurally characterized by 1D and 2D NMR, allowing an elucidation of the formation mechanism of these oaklin-catechin adducts and demonstrating the importance of procyanidins in the formation of colored compounds through the reaction with cinnamic aldehydes extracted from oaks during storage.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
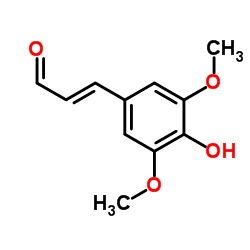 |
反-3,5-二甲氧基-4-羟基肉桂醛
CAS:4206-58-0 |
C11H12O4 |
|
[Study on chemical constituents from ethyl acetate extract o...
2011-04-01 [Zhongguo Zhong Yao Za Zhi 36(8) , 1019-23, (2011)] |
|
A rapid sample screening method for authenticity control of ...
2011-07-13 [J. Agric. Food Chem. 59(13) , 6882-8, (2011)] |
|
Influences of cinnamic aldehydes on H⁺ extrusion activity an...
2013-02-01 [J. Med. Microbiol. 62(Pt 2) , 232-40, (2013)] |
|
GC/MS-positive ion chemical ionization and MS/MS study of vo...
2007-05-01 [J. Mass Spectrom. 42(5) , 641-6, (2007)] |
|
The last step of syringyl monolignol biosynthesis in angiosp...
2001-07-01 [Plant Cell 13(7) , 1567-86, (2001)] |