Enzymic oxidation of capsaicin.
A Boersch, B A Callingham, F Lembeck, D F Sharman
文献索引:Biochem. Pharmacol. 41(12) , 1863-9, (1991)
全文:HTML全文
摘要
The oxidation of capsaicin (8-methyl-N-vanillyl-6-nonenamide) has been investigated by means of electrochemical, enzymic and chemical procedures. Capsaicin appears to form a fluorescent dimer comparable with those known to be formed from some other compounds bearing the vanillyl(4-hydroxy-3-methoxybenzyl-) group. If such a dimer of capsaicin were to be formed in tissues, it would bind tightly to lipid structures and its formation would prove difficult to follow. Tests on other substances bearing the vanillyl group that might be used to investigate the dimerization reaction in tissues and tissue extracts showed that 4-hydroxy-3-methoxyphenylacetic acid is a poor second substrate for peroxidase reactions. It was found that 2-methoxy-4-methylphenol (creosol) was more suitable. These results support the suggestion that the oxidation of capsaicin may be involved in some of its biological actions.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
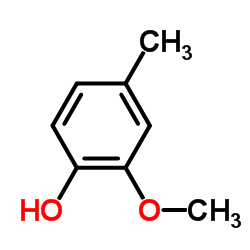 |
2-甲氧基-4-甲基苯酚
CAS:93-51-6 |
C8H10O2 |
|
In chemico evaluation of prohapten skin sensitizers: behavio...
2013-04-26 [Toxicol. Lett. 218(3) , 266-72, (2013)] |
|
Rapid discrimination and characterization of vanilla bean ex...
2012-03-01 [J. Food Sci. 77(3) , C284-92, (2012)] |
|
Binding of alkyl- and alkoxy-substituted simple phenolic com...
2000-01-01 [Res. Commun. Mol. Pathol. Pharmacol. 107(1-2) , 167-73, (2000)] |
|
Thermal stabilization of levoglucosan in aromatic substances...
2006-09-25 [Carbohydr. Res. 341(13) , 2293-7, (2006)] |
|
Urinary excretion of phenols as an indicator of occupational...
1997-01-01 [Int. Arch. Occup. Environ. Health 70(5) , 334-40, (1997)] |