2,4'-Dihydroxyacetophenone dioxygenase (EC 1.13.11.41) from Alcaligenes sp. 4HAP: a novel enzyme with an atypical dioxygenase sequence.
D J Hopper, M A Kaderbhai
文献索引:Biochem. J. 344 Pt 2 , 397-402, (1999)
全文:HTML全文
摘要
2,4'-Dihydroxyacetophenone dioxygenase (EC 1.13.11.41) was purified to homogeneity from Alcaligenes sp. 4HAP grown on 4-hydroxyacetophenone. Measurements of the M(r) of the native enzyme ranged from 81600 to 87000, whereas values of 21000 and 20379 were given by SDS/PAGE and electrospray MS respectively. The enzyme is a homotetramer and contains one atom of iron per molecule of enzyme. From C- and N-terminal analyses, primers for PCR were designed and the dad gene cloned and sequenced. The predicted amino acid sequence of dad, deduced from the nucleotide sequence, confirms the N-terminal amino acid sequencing data and contains the sequence of an internal tryptic peptide. It gave a calculated M(r) of 20364. The gene was expressed in Escherichia coli and yielded active enzyme. The derived amino acid sequence does not show significant similarity to other dioxygenases or any strong similarity to protein sequences presently available in the databases.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
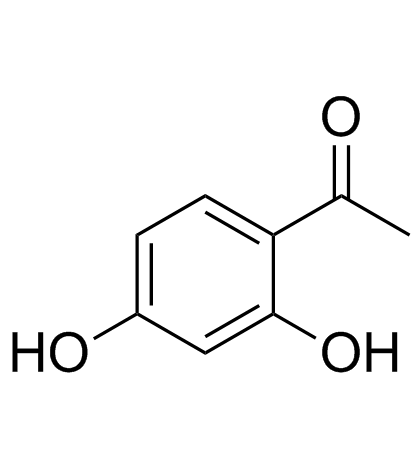 |
2',4'-二羟基苯乙酮
CAS:89-84-9 |
C8H8O3 |
|
Bioactive metabolites from biotransformation of paeonol by t...
2011-08-01 [Nat. Prod. Commun. 6(8) , 1129-30, (2011)] |
|
Multifaceted defense against antagonistic microbes in develo...
2014-01-01 [PLoS ONE 9(6) , e98784, (2014)] |
|
Synthesis, Characterization and Biological Studies of Metal(...
2015-01-01 [Molecules 20 , 9788-802, (2015)] |
|
Aromatic aldehydes and aromatic ketones open ATP-sensitive K...
1992-08-01 [Pflugers Arch. 421(5) , 409-15, (1992)] |
|
Application of high-resolution ESI and MALDI mass spectromet...
2012-08-01 [J. Mass Spectrom. 47(8) , 1015-22, (2012)] |