Characterization of two novel tritiated radioligands for labelling Neuropeptide FF (NPFF1and NPFF2) receptors
Franck Talmont, Laura Piedra Garcia, Honoré Mazarguil, Jean-Marie Zajac, Catherine Mollereau, Franck Talmont, Laura Piedra Garcia, Honoré Mazarguil, Jean-Marie Zajac, Catherine Mollereau
文献索引:Neurochem. Int. 55(8) , 815-9, (2009)
全文:HTML全文
摘要
The binding characteristics of [ 3H]-NPVF and [ 3H]-EYF, the two first tritiated probes for the respective labelling of NPFF 1 and NPFF 2 receptors, are presented. In membranes from CHO cells transfected with the human NPFF 1 receptor, [ 3H]-NPVF labelled one class of binding sites with a high affinity (Bmax = 4 pmol/mg protein, Kd = 2.65 nM). In membranes from CHO cells transfected with the human NPFF 2 receptor, [ 3H]-EYF labelled one class of binding sites with a high affinity (Bmax = 16 pmol/mg protein, Kd = 0.54 nM). Both radioligands exhibited time-dependent binding, low (10–20%) non-specific binding and poor cross-reactivity towards the related receptor subtype. The potency of different NPFF ligands to displace [ 3H]-NPVF and [ 3H]-EYF binding profiles was in good agreement with the profile previously measured by using 125I-probes (NPFF 1 receptor: NPVF ≥ 1DMe = SPA-NPFF > NPFF = SQA-NPFF = QFW-NPSF > NPSF > RF9; NPFF 2 receptor: SPA-NPFF > > SQA-NPFF = QFW-NPSF = 1DMe = NPFF ≫ NPSF = NPVF > RF9). Therefore, [ 3H]-NPVF and [ 3H]-EYF are new valuable tools for performing binding on NPFF receptors.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
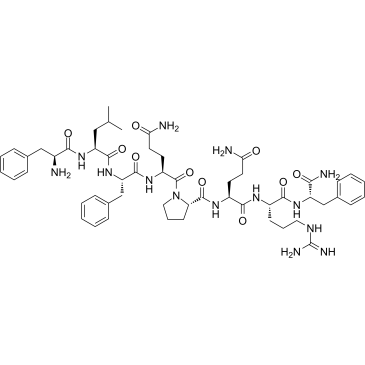 |
Neuropeptide FF trifluoroacetate salt
CAS:99566-27-5 |
C54H76N14O10 |
|
The anti-inflammatory potential of neuropeptide FF in vitro ...
2013-09-01 [Peptides 47 , 124-32, (2013)] |
|
Intracerebroventricular administration of 26RFa produces an ...
2009-09-01 [Peptides 30(9) , 1683-8, (2009)] |
|
Pressor and tachycardic responses to intrathecal administrat...
2010-04-01 [Peptides 31(4) , 683-8, (2010)] |
|
Nonpeptide ligands of neuropeptide FF: current status and st...
2012-06-01 [Future Med. Chem. 4(9) , 1085-92, (2012)] |
|
Opposite control of body temperature by NPFF1 and NPFF2 rece...
2010-10-01 [Neuropeptides 44(5) , 453-6, (2010)] |