A synthetic endopeptidase substrate hydrolyzed by the bovine lens neutral proteinase preparation.
B J Wagner, S C Fu, J W Margolis, K R Fleshman
文献索引:Exp. Eye Res. 38(5) , 477-83, (1984)
全文:HTML全文
摘要
Lens neutral proteinase is thought to exhibit primarily endopeptidase activity. We have identified a synthetic endopeptidase substrate which is hydrolyzed by the bovine lens neutral proteinase preparation. Among 11 fluoro- and chromogenic endopeptidase substrates, only carbobenzoxy-glycylglycyl-L-leucyl-p-nitroanilide is effectively hydrolyzed. The activity hydrolyzing this substrate co-elutes with neutral proteinase activity upon gel filtration and specifically attacks the leucyl-p-nitroaniline bond. Optimal hydrolysis of the synthetic substrate is at neutral pH and high temperature (53 degrees C), analogous to the alpha-crystallin protein substrate obtained from lens. The rate of hydrolysis of the synthetic substrate increased proportionally with temperature between 20 and 60 degrees C, in contrast to alpha-crystallin. The rate of hydrolysis was linear for at least 1 h at 37 degrees C and there was no evidence of enzyme activation at high temperature.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
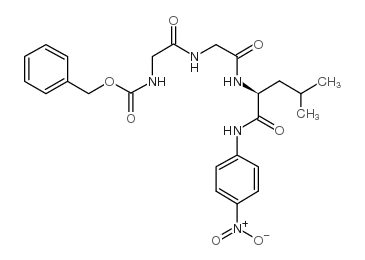 |
Z-GLY-GLY-LEU-PNA
CAS:53046-98-3 |
C24H29N5O7 |
|
A new chromogenic substrate for subtilisin.
1974-12-01 [Anal. Biochem. 62 , 371-376, (1974)] |
|
Degradation of bradykinin by isolated neutral endopeptidases...
1979-09-12 [Biochem. Biophys. Res. Commun. 90 , 1, (1979)] |
|
Evidence that pituitary cation-sensitive neutral endopeptida...
1983-03-01 [J. Neurochem. 40 , 842-849, (1983)] |
|
Activity of Subtilisin Carlsberg in macromolecular crowding.
2007-03-01 [J. Photochem. Photobiol. B, Biol. 86(3) , 199-206, (2007)] |
|
Technical note: quantification of multicatalytic proteinase ...
1993-12-01 [J. Anim. Sci. 71(12) , 3301-6, (1993)] |