Oxazolidonylethyl adducts to hemoglobin and DNA following nornitrogen mustard exposure.
H Thulin, V Zorcec, D Segerbäck, A Sundwall, M Törnqvist
文献索引:Chem. Biol. Interact. 99(1-3) , 263-75, (1996)
全文:HTML全文
摘要
Formation of adducts to hemoglobin (Hb) and DNA of nornitrogen mustard (NNM) was studied with the aim of developing a method for monitoring exposure to NNM. Adducts to N-terminal valines in Hb were studied by the N-alkyl Edman method using pentafluorophenyl isothiocyanate (PFPITC) as the derivatizing reagent. In preliminary studies five major Hb adducts were shown to be formed in reaction of NNM with red cell hemolysate in vitro. Following treatment with PFPITC three of these were found to be pentafluorophenylthiohydantoins (PFPTHs) of N-alkylated valines and the fourth probably originates from NNM esters in which PFPITC had reacted with the nitrogen of N-chloroethylaminoethyl. A PFPTH was found to originate from N-2-(3-oxazolidonyl)ethylvaline, Val-OZ. Val-OZ is formed in reaction, with ring closure to oxazolidone, of CO2 with the 2-chloroethylamino group in the primary valine-N adduct. Besides a few other adducts, Val-OZ was also observed in mouse Hb following injection of NNM, and also after injection of cyclophosphamide. Following reaction in vitro of NNM with DNA, three major adducts to guanine-N-7 were observed; one of them, 7-(N'-(2-chloroethyl)-2-aminoethyl]-guanine (NNMCl), was converted by carbonate to 7-(2-3-oxazolidonyl)ethyl]guanine (Gua-OZ). In mice treated with NNM, Gua-Oz was the only DNA adduct observed. Val-Oz is a chemically stable Hb adduct, potentially useful for monitoring exposures to NNM and cyclophosphamide.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
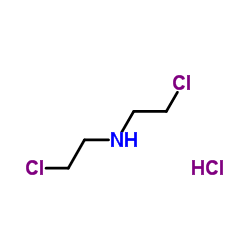 |
二(2-氯乙基)胺盐酸盐
CAS:821-48-7 |
C4H10Cl3N |
|
Occupational exposure to nor-nitrogen mustard: chemical and ...
1995-01-01 [Toxicol. Ind. Health 11(1) , 89-97, (1995)] |
|
Basic principles in preclinical cancer chemotherapy.
1990-01-01 [J. Cancer Res. Clin. Oncol. 116(5) , 411-24, (1990)] |
|
Covalent binding of nitrogen mustards to the cysteine-34 res...
2002-03-01 [Arch. Toxicol. 76(2) , 83-8, (2002)] |
|
The determination of cyclophosphamide and its metabolites in...
1994-03-01 [Biol. Mass Spectrom. 23(3) , 149-58, (1994)] |
|
The effect of estramustine, nor-nitrogen mustard and tauromu...
1989-01-01 [Pharmacol. Toxicol. 64(1) , 9-13, (1989)] |