Characterization and primary structure of proteins L28, L33 and L34 from Bacillus stearothermophilus ribosomes.
V Kruft, U Kapp, B Wittmann-Liebold
文献索引:Biochimie 73(7-8) , 855-60, (1991)
全文:HTML全文
摘要
The complete amino acid sequences of 3 proteins from the 50S subunit of Bacillus stearothermophilus ribosomes were determined by N-terminal sequence analysis and by sequencing of overlapping fragments obtained from enzymatic digestions and chemical cleavages. The proteins BstL28, BstL33 and BstL34, named according to the equivalent proteins in Escherichia coli ribosomes, consist of 60, 49, and 44 amino acid residues and have calculated molecular masses of 6811.0, 5908.6, and 5253.9 Da, respectively. They are highly basic with a content of positively charged residues ranging between 29% for L33 and 45% for L34. The 3 proteins were positioned in the 2-dimensional map of B stearothermophilus 50S ribosomal proteins. The electrophoretic mobilities confirm sizes and net charges deduced from the sequences.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
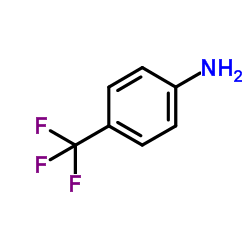 |
4-氨基三氟甲苯
CAS:455-14-1 |
C7H6F3N |
|
The many roles for fluorine in medicinal chemistry.
2008-08-14 [J. Med. Chem. 51 , 4359-69, (2008)] |
|
Trifluoromethylanilines--their effect on DNA synthesis and p...
1993-10-25 [Toxicology 83(1-3) , 49-59, (1993)] |
|
Effects of trifluoromethylaniline isomers on enzyme activiti...
1994-09-06 [Toxicology 92(1-3) , 27-38, (1994)] |
|
Mapping of the human ribosomal small subunit protein gene RP...
1997-01-01 [Genomics 39(1) , 121-2, (1997)] |
|
Differential display and cloning of messenger RNAs from the ...
1995-07-06 [Biochem. Biophys. Res. Commun. 212(1) , 21-6, (1995)] |