| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
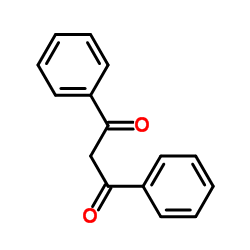 |
二苯甲酰甲烷
CAS:120-46-7 |
|
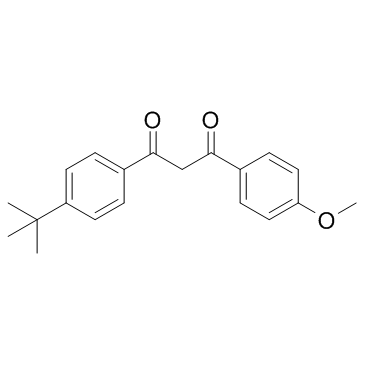 |
阿伏苯宗
CAS:70356-09-1 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
二苯甲酰甲烷
CAS:120-46-7 |
|
 |
阿伏苯宗
CAS:70356-09-1 |