New continuous and specific fluorometric assays for Pseudomonas aeruginosa elastase and LasA protease.
Caroline Elston, Jean Wallach, Joëlle Saulnier
文献索引:Anal. Biochem. 368(1) , 87-94, (2007)
全文:HTML全文
摘要
A highly sensitive assay based on new internally quenched fluorogenic peptide substrates has been developed for monitoring protease activities. These novel substrates comprise an Edans (5-(2-aminoethylamino)-1-naphthalenesulfonic acid) group at the C terminus and a Dabsyl (4-(dimethylamino)azobenzene-4'-sulfonyl chloride) fluorophore at the N terminus of the peptide chains. The Edans fluorescence increases upon peptide hydrolysis by Pseudomonas aeruginosa proteases, and this increase is directly proportional to the amount of substrate cleaved, i.e., protease activity. The substrates Dabsyl-Ala-Ala-Phe-Ala-Edans and Dabsyl-Leu-Gly-Gly-Gly-Ala-Edans were used for testing the peptidasic activities of P. aeruginosa elastase and LasA protease, respectively. Elastase and LasA kinetic parameters were calculated and a sensitive assay was designed for the detection of P. aeruginosa proteases in bacterial supernatants. The sensitivity and the small sample requirements make the assay suitable for high-throughput screening of biological samples. Furthermore, this P. aeruginosa protease assay improves upon existing assays because it is simple, it requires only one step, and even more significantly it is enzyme specific.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
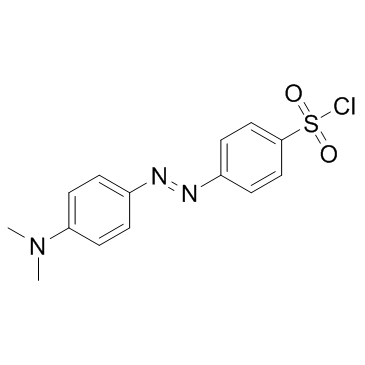 |
4-二甲胺基苯基偶氮苯磺酰氯
CAS:56512-49-3 |
C14H14ClN3O2S |
|
A derivatization and validation strategy for determining the...
2014-08-01 [J. Mass Spectrom. 49(8) , 665-73, (2014)] |
|
Lysine-directed staining of proteins for MS-based analyses.
2013-02-01 [Electrophoresis 34(3) , 401-4, (2013)] |
|
Spectrophotometric determination of bacitracin in bulk drug ...
2011-01-01 [Acta Pol. Pharm. 68(6) , 853-8, (2011)] |
|
Fast liquid chromatography/tandem mass spectrometry determin...
2012-09-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 905 , 85-95, (2012)] |
|
Rapid determination of dabsylated hydroxyproline from cultur...
1995-02-17 [J. Chromatogr. B, Biomed. Appl. 664(2) , 435-9, (1995)] |