4-fluoro-2-methoxyphenol, an apocynin analog with enhanced inhibitory effect on leukocyte oxidant production and phagocytosis.
Ana Carolina de Almeida, Otávio Cabral Marques, Christina Arslanian, Antonio Condino-Neto, Valdecir F Ximenes
文献索引:Eur. J. Pharmacol. 660(2-3) , 445-53, (2011)
全文:HTML全文
摘要
Apocynin, a methoxy-substituted catechol (4-hydroxy-3-methoxyacetophenone), originally extracted from the roots of Picrorhiza kurroa, has been extensively used as a non-toxic inhibitor of the multienzymatic complex NADPH oxidase. We discovered that the analogous methoxy-substituted catechol, 4-Fluoro-2-methoxyphenol (F-apocynin), in which the acetyl group present in apocynin was changed to a fluorine atom, was significantly more potent as an inhibitor of NADPH oxidase activity, myeloperoxidase (MPO) chlorinating activity and phagocytosis of microorganisms by neutrophils; it was also as potent as apocynin in inhibiting tumor necrosis factor-alpha (TNFα) release by peripheral blood mononuclear cells. We attribute the increased potency of F-apocynin to its increased lipophilicity, which could facilitate the passage of the drug through the cell membrane. The inhibition of MPO chlorination activity, phagocytosis and TNFα release shows that apocynin and F-apocynin actions are not restricted to reactive oxygen species inhibition, but further studies are needed to clarify if these mechanisms are related. Like apocynin, F-apocynin did not show cell toxicity, and is a strong candidate for use in the treatment of inflammatory diseases.Copyright © 2011 Elsevier B.V. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
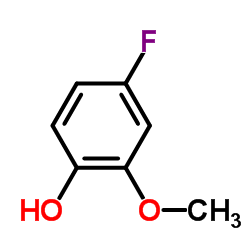 |
4-氟-2-甲氧基苯酚
CAS:450-93-1 |
C7H7FO2 |
|
Oxidation of substituted 4-fluorobenzaldehydes: application ...
1991-01-01 [Int. J. Rad. Appl. Instrum. A. 42(7) , 673-81, (1991)] |
|
Diels-Alder reactions of halogenated masked o-benzoquinones:...
[Tetrahedron Lett. 50(7) , 773-775, (2009)] |
|
A fluorinated masked o-benzoquinone. Patrick TB, et al.
[J. Fluor. Chem. 125(12) , 1965-1966, (2007)] |
|
Novel photoconductive polyfluorophenol synthesized by an enz...
[J. Mol. Catal., B Enzym. 72(1) , 25-27, (2011)] |