Integration of newly isolated biocatalyst and resin-based in situ product removal technique for the asymmetric synthesis of (R)-methyl mandelate.
Jin-Ling Guo, Xiao-Qing Mu, Yan Xu
文献索引:Bioprocess Biosyst. Eng. 33(7) , 797-804, (2010)
全文:HTML全文
摘要
The enantioselective reduction of methyl benzoylformate to (R)-methyl mandelate, an important pharmaceutical intermediate and a versatile resolving agent, was investigated in this study. After minimizing the reaction-specific constraints (constraints dependent on the nature of the substrate and product) by preliminary selection of the reaction parameters, an effective whole cell biocatalyst (Saccharomyces cerevisiae AS2.1392) was obtained by simple screening procedures. Under further optimized conditions, a product concentration of 103 mmol L(-1) could be attained within 5 h with a yield of 85.8% and an enantiometric excess of 95.4%, indicating S. cerevisiae AS2.1392 an efficient biocatalyst for the asymmetric synthesis of (R)-methyl mandelate. Furthermore, resin-based in situ product removal (ISPR) technique was applied to alleviate the substrate and product inhibition or toxicity to the whole cells. The integration of newly isolated biocatalyst and proper ISPR technique provides a practical route for the preparation of optically active pharmaceutical intermediates.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
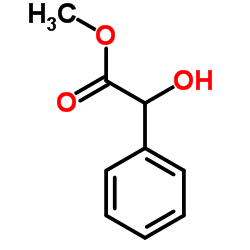 |
DL-扁桃酸甲酯
CAS:4358-87-6 |
C9H10O3 |
|
Hydrolysis of triacetin catalyzed by immobilized lipases: Ef...
2011-01-01 [Enzyme Microb. Technol. 48 , 510-517, (2011)] |
|
Esters of mandelic acid as substrates for (S)-mandelate dehy...
2004-02-24 [Biochemistry 43(7) , 1883-90, (2004)] |
|
Generation of the enol of methyl mandelate by flash photolys...
2000-02-25 [J. Org. Chem. 65(4) , 1175-80, (2000)] |
|
Theoretical study on chiral recognition mechanism of methyl ...
2012-02-01 [J. Mol. Model. 18(2) , 803-13, (2012)] |
|
Calculations of the energy distribution from perturbation pe...
2008-01-01 [J. Chromatogr. A. 1203(2) , 177-84, (2008)] |