Activation of a GH43 β-xylosidase by divalent metal cations: slow binding of divalent metal and high substrate specificity.
Douglas B Jordan, Charles C Lee, Kurt Wagschal, Jay D Braker
文献索引:Arch. Biochem. Biophys. 533(1-2) , 79-87, (2013)
全文:HTML全文
摘要
RS223-BX of glycoside hydrolase family 43 is a β-d-xylosidase that is strongly activated (k(cat)/K(m) as much as 116-fold) by the addition of divalent metal cations, Ca(2+), Co(2+), Fe(2+), Mg(2+), Mn(2+) and Ni(2+). Slow activation by Mg(2+) was demonstrated (k(on) 0.013 s(-1) mM(-1), k(off) 0.008 s(-1)) at pH 7.0 and 25 °C. k(off) and k(on) values are independent of Mg(2+) concentration, but k(off) and k(on) are slower in the presence of increasing levels of substrate 4-nitrophenyl-β-D-xylopyranoside. The kinetics strongly suggest that M(2+) binds to the enzyme rapidly, forming E M(2+), followed by slow isomerization to the activated enzyme, E* M(2+). Moderately high values of kcat (7-30 s(-1)) were found for M(2+)-activated RS223-BX acting on xylobiose (natural substrate) at pH 7.0 and 25 °C. Certain M(2+)-activated RS223-BX exhibit the highest reported values of k(cat)/K(m) of any β-xylosidase acting on natural substrates: for example, at pH 7.0 and 25°C, xylobiose (Mn(2+), 190 s(-1) mM(-1)), xylotriose (Ca(2+), 150 s(-1) mM(-1)) and xylotetraose (Ca(2+), 260 s(-1) mM(-1)). There is potential for the enzyme to add value to industrial saccharification operations at low substrate and high d-glucose and high d-xylose concentrations.Published by Elsevier Inc.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
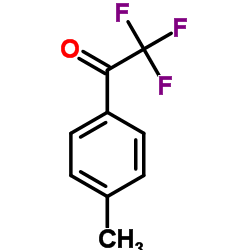 |
Exo-1,4-β-xylosidase
CAS:9025-53-0 |
C9H7F3O |
|
A novel xylan degrading β-D-xylosidase: purification and bio...
2012-11-01 [World J. Microbiol. Biotechnol. 28(11) , 3179-86, (2012)] |
|
Beta-xylosidase activity of a GH3 glucosidase/xylosidase fro...
2012-02-01 [Lett. Appl. Microbiol. 54(2) , 79-87, (2012)] |
|
Purification and characterization of a recombinant β-D-xylos...
[Protein J. 31(8) , 641-50, (2012)] |
|
Biochemical and kinetic characterization of GH43 β-D-xylosid...
2012-01-01 [J. Ind. Microbiol. Biotechnol. 39(1) , 143-52, (2012)] |
|
Two xylose-tolerant GH43 bifunctional β-xylosidase/α-arabino...
2014-04-01 [Food Chem. 148 , 381-7, (2014)] |