Biotinyl-methyl 4-(amidomethyl)benzoate is a competitive inhibitor of human biotinidase.
Keyna A Kobza, Kittichai Chaiseeda, Gautam Sarath, James M Takacs, Janos Zempleni
文献索引:J. Nutr. Biochem. 19 , 826-832, (2008)
全文:HTML全文
摘要
Posttranslational modification of histones by biotinylation can be catalyzed by both biotinidase (BTD) and holocarboxylase synthetase. Biotinylation of histones is an important epigenetic mechanism to regulate gene expression, DNA repair, and chromatin remodeling. The role of BTD in histone biotinylation is somewhat ambiguous, given that BTD also catalyzes removal of the biotin tag from histones. Here, we sought to develop BTD inhibitors for future studies of the role of BTD in altering chromatin structure. We adopted an existing colorimetric BTD assay for use in a novel 96-well plate format to permit high-throughput screening of potential inhibitors. Biotin analogs were chemically synthesized and tested for their ability to inhibit human BTD. Seven of these compounds inhibited BTD by 26-80%. Biotinyl-methyl 4-(amidomethyl)benzoate had the largest effect on BTD, causing an 80% inhibition at 1 mM concentration. Enzyme kinetics studies were conducted to determine V(max), K(m) and K(i) for the seven inhibitors; kinetics were consistent with the hypothesis that biotinyl-methyl 4-(amidomethyl)benzoate and the other compounds acted by competitive inhibition of BTD. Finally, biotinyl-methyl 4-(amidomethyl)benzoate did not affect biotin transport in human cells, suggesting specificity in regard to biotin-related processes.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
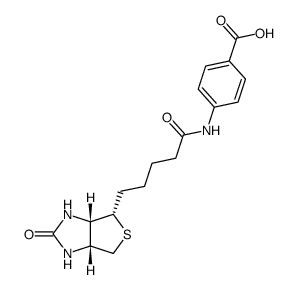 |
N-(+)-生物素基-4-氨基苯甲酸
CAS:6929-40-4 |
C17H21N3O4S |
|
Neonatal screening for biotinidase deficiency.
1992-01-01 [Ann. Clin. Lab. Sci. 22 , 144-154, (1992)] |
|
Determination of biotinidase activity by liquid chromatograp...
[J. Chromatogr. A. 383 , 148, (1986)] |
|
Purification of biotinidase from human plasma and its activi...
1985-05-07 [Biochemistry 24 , 2471, (1985)] |