NADH oxidation by quinone electron acceptors.
N K Cénas, J J Kanapieniené, J J Kulys
文献索引:Biochim. Biophys. Acta 767(1) , 108-12, (1984)
全文:HTML全文
摘要
The rate constants of NADH oxidation by quinones are increased with the oxidation potential increase: log kox (M-1 X s-1) = -0.25 + 12.2 E0(7) (V) for o-quinones and log kox (M-1 X s-1) = -3.06 + 13.5 E0(7) (V) for p-quinones (pH 7.0, 25 degrees C). It is assumed that the oxidation proceeds via the hydride-ion transfer. The rate constants of NADH oxidation by single-electron quinone acceptors are also increased with the oxidizer potential increase; log kox (M-1 X s-1) = -0.64 + 9.34 E0(7) (V) and correlate with the constants of NADH oxidation by quinone radicals obtained earlier (Grodkowski, J., Neta, P., Carlson, B.W. and Miller, L. (1983) J. Phys. Chem. 87, 3135-3138). Single-electron transfer is the limiting stage of the process.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
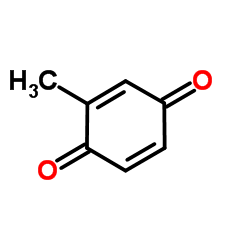 |
甲基苯醌
CAS:553-97-9 |
C7H6O2 |
|
Indoleamine 2,3-dioxygenase is the anticancer target for a n...
2008-03-27 [J. Med. Chem. 51 , 1706-18, (2008)] |
|
A class of benzenoid chemicals suppresses apoptosis in C. el...
2006-12-01 [ChemBioChem. 7(12) , 2010-5, (2006)] |
|
Oxidative DNA damage by minor metabolites of toluene may lea...
1999-08-02 [Biochem. Biophys. Res. Commun. 261(2) , 478-83, (1999)] |
|
Isolation and characterizations of quinone analogue-resistan...
1998-09-15 [Biochemistry 37(37) , 12744-52, (1998)] |
|
Odoriferous Defensive stink gland transcriptome to identify ...
2013-01-01 [PLoS Genet. 9(7) , e1003596, (2013)] |