Electrophilic fluorination of pyroglutamic acid derivatives: application of substrate-dependent reactivity and diastereoselectivity to the synthesis of optically active 4-fluoroglutamic acids.
D W Konas, J K Coward
文献索引:J. Org. Chem. 66(26) , 8831-42, (2001)
全文:HTML全文
摘要
Electrophilic fluorination of enantiomerically pure 2-pyrrolidinones (4) derived from (L)-glutamic acid has been investigated as a method for the synthesis of single stereoisomers of 4-fluorinated glutamic acids. Reaction of the lactam enolate derived from 9 with NFSi results in a completely diastereoselective monofluorination reaction to yield the monocyclic trans-substituted alpha-fluoro lactam product 21. Unfortunately, a decreased kinetic acidity in 21 and other structurally related monofluorinated products renders them resistant to a second fluorination. In contrast, the bicyclic lactam 12 is readily difluorinated under the standard conditions described to yield the alpha,alpha-difluoro lactam 24. The difference in reactivity between the two types of related lactams is attributed mainly to the presence or lack of a steric interaction between the base used for deprotonation and the protecting group present in the pyrrolidinone substrates. This conclusion was reached based on analysis of the X-ray crystal structure of 21, molecular modeling, and experimental evidence. The key intermediates 21 and 24 are converted to (2S,4R)-4-fluoroglutamic acid and (2S)-4,4-difluoroglutamic acid, respectively.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
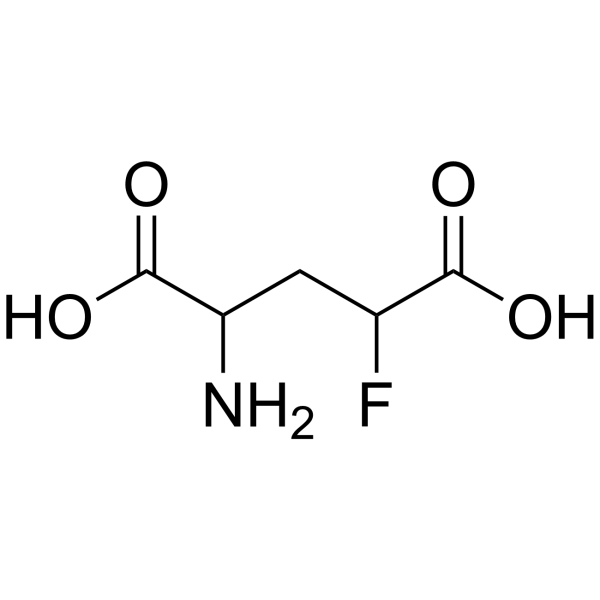 |
4-氟-DL-谷氨酸
CAS:2708-77-2 |
C5H8FNO4 |
|
4-[18F]fluoroglutamic acid (BAY 85-8050), a new amino acid r...
2011-01-13 [J. Med. Chem. 54(1) , 406-10, (2011)] |
|
Mutations of the Corynebacterium glutamicum NCgl1221 gene, e...
2007-07-01 [Appl. Environ. Microbiol. 73(14) , 4491-8, (2007)] |
|
Vitamin K-dependent carboxylation. Study of the hydrogen abs...
1983-07-10 [J. Biol. Chem. 258(13) , 7897-9, (1983)] |
|
Glutamylation of methotrexate in hepatoma cells in vitro: re...
1985-01-01 [Adv. Enzyme Regul. 23 , 13-23, (1985)] |
|
Bacterial defluorination of 4-fluoroglutamic acid.
2007-12-01 [Appl. Microbiol. Biotechnol. 77(3) , 699-703, (2007)] |