Interaction of oxytocin with Ca2+: I. CD and fluorescence spectral characterization and comparison with vasopressin.
V S Ananthanarayanan, K S Brimble
文献索引:Biopolymers 40(5) , 433-43, (1996)
全文:HTML全文
摘要
Extracellular Ca2+ is required for the action of oxytocin and both the hormone and its receptor have binding sites for divalent metal cations. To characterize the cation-bound form of oxytocin, we monitored the binding of Ca2+ and Mg2+ to oxytocin as well as peptides representing its ring and tail regions in trifluoroethanol, a lipid-mimetic solvent, using CD and fluorescence spectroscopy. Binding Ca2+ (Kd approximately 50 microM) caused drastic CD and fluorescence changes leading to a helical conformation. Mg2+ caused CD changes smaller than and opposite to Ca2+. However, the helical structure was enhanced when both Ca2+ and Mg2+ were present together. CD changes in the tail peptide of oxytocin showed its ability to bind Ca2+ and Mg2+ whereas the vasopressin tail peptide did not bind either cation. CD spectral changes on Ca2+ and Mg2+ binding to tocinoic acid (the ring moiety of oxytocin) were much smaller than those of oxytocin. These data suggest that the tail segment of oxytocin potentiates Ca2+ binding by the ring. While vasopressin displayed a CD spectrum similar to that of oxytocin, CD spectra of its cation-bound forms were markedly different from those of oxytocin; the Ca(2+)-induced CD changes in vasopressin were very much smaller and of opposite sign, and Mg(2+)-induced ones significantly larger than in oxytocin. Taken together, our observations bring out the structural differences between oxytocin and vasopressin in the context of their interaction with Ca2+ and Mg2+. This may be relevant to understanding the differences in the bioactive conformations and receptor interactions of the two hormones.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
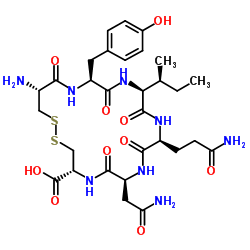 |
CYS-TYR-ILE-GLN-ASN-CYS: CYIQNCDISULFIDE BRIDGE CYS1-CYS6
CAS:34330-23-9 |
C30H44N8O10S2 |
|
The role of oxytocin release in the paraventricular nucleus ...
1996-03-01 [J. Neuroendocrinol. 8 , 163, (1996)] |
|
Prolactin-releasing activity of neurohypophysial hormones: s...
1994-01-01 [Endocrinology 134 , 114, (1994)] |
|
Oxytocin-like peptide: a novel epitope colocalized with the ...
1993-01-22 [Brain Res. 601 , 173, (1993)] |
|
[Effect of arginine vasotocin, oxytocin and their fragments ...
1982-01-01 [Fiziol. Zh. SSSR Im. I. M. Sechenova 68(1) , 112-5, (1982)] |
|
Hypothalamic oxytocin mediates adaptation mechanism against ...
2010-10-01 [Am. J. Physiol. Gastrointest. Liver Physiol. 299(4) , G946-53, (2010)] |