Structural mechanism of ribonucleotide discrimination by a Y-family DNA polymerase.
Kevin N Kirouac, Zucai Suo, Hong Ling
文献索引:J. Mol. Biol. 407 , 382-390, (2011)
全文:HTML全文
摘要
The ability of DNA polymerases to differentiate between ribonucleotides and deoxribonucleotides is fundamental to the accurate replication and maintenance of an organism's genome. The active sites of Y-family DNA polymerases are highly solvent accessible, yet these enzymes still maintain a high selectivity towards deoxyribonucleotides. Here, we biochemically demonstrate that a single active-site mutation (Y12A) in Dpo4, a model Y-family DNA polymerase, causes both a dramatic loss of ribonucleotide discrimination and a decrease in nucleotide incorporation efficiency. We also determined two ternary crystal structures of the Dpo4 Y12A mutant incorporating either dATP or ATP nucleotides opposite a template dT base. Interestingly, both dATP and ATP were hydrolyzed to dADP and ADP, respectively. In addition, the dADP and ADP molecules adopt a similar conformation and position at the polymerase active site to a ddADP molecule in the ternary crystal structure of wild-type Dpo4. The Y12A mutant loses stacking interactions with the deoxyribose of dNTP, which destabilizes the binding of incoming nucleotides. The mutation also opens a space to accommodate the 2'-OH group of the ribose of NTP in the polymerase active site. The structural change leads to the reduction in deoxynucleotide incorporation efficiency and allows ribonucleotide incorporation.Copyright © 2011 Elsevier Ltd. All rights reserved.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
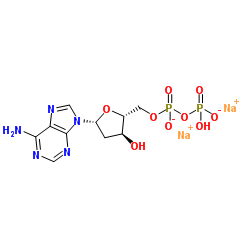 |
2ˊ-脱氧腺苷 5ˊ-二磷酸钠盐
CAS:72003-83-9 |
C10H13N5Na2O9P2 |
|
Substrate specificity and nucleotides binding properties of ...
2010-10-01 [J. Bioenerg. Biomembr. 42 , 361-369, (2010)] |
|
Enzymatic synthesis of oligonucleotides of defined sequence....
1975-05-01 [Nucleic Acids Res. 2 , 613-624, (1975)] |
|
Synthesis and degradation of poly(A) in permeable cells of E...
1978-08-25 [J. Biol. Chem. 253 , 5579-5584, (1978)] |