Inhibition of mitochondrial carnitine-acylcarnitine translocase by sulfobetaines.
R Parvin, T Goswami, S V Pande
文献索引:Can. J. Biochem. 58 , 822, (1980)
全文:HTML全文
摘要
Sulfobetaines (N-alkyl-N,N-dimethyl-3-ammonio-1-propanesulfonates) have been identified as relatively specific and selective inhibitors of mitochondrial carnitine-acylcarnitine translocase. Thus, sublytic concentrations of sulfobetaines (alkyl = octyl to tetradecyl) inhibit the respiration of rat heart mitochondria supported by added acylcarnitines or pyruvate plus malonate and carnitine. Both exchange efflux and unidirectional net efflux of mitochondrial carnitine are also inhibited; the half-maximal inhibition of the former occurs at micromolar concentrations of sulfobetaines and the inhibitory effect is reversible and competitive with respect to carnitine. As a stop-inhibitor, 20 mM sulfobetaine, (alkyl = octyl), is useable at near 0 degrees C but is less effective than 2 mM mersalyl when transport rates are very rapid as at higher temperatures especially with liver mitochondria. The loss of mitochondrial carnitine that normally occurs owing to the progress of net efflux during the isolation of mitochondria is prevented by the inclusion of 20 mM sulfobetaine in the isolation medium and this enables a better estimate of the mitochondrial carnitine content. Sulfobetaines inhibit the activities of mitochondrial carnitine acetyltransferase and carnitine palmitoyltransferase but only at concentrations severalfold higher than those inhibitory for the translocase. This observation supports the belief that carnitine-acylcarnitine translocase is an entity distinct from that of carnitine acyltransferases.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
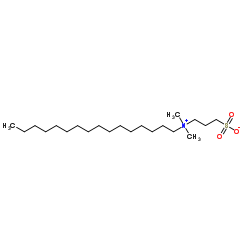 |
3-磺丙基十六烷基二甲基铵
CAS:2281-11-0 |
C21H45NO3S |
|
Multi-target spectral moment QSAR versus ANN for antiparasit...
2010-03-15 [Bioorg. Med. Chem. 18 , 2225-31, (2010)] |
|
Highly sensitive analysis of flavonoids by zwitterionic micr...
2014-09-05 [J. Chromatogr. A. 1358 , 277-84, (2014)] |
|
Further characterization of rat dihydroceramide desaturase: ...
2000-10-01 [Lipids 35 , 1117, (2000)] |
|
Solubilization and partial purification of retinyl ester syn...
1989-06-05 [J. Biol. Chem. 264 , 9231, (1989)] |
|
Examination of micro-tip reversed-phase liquid chromatograph...
1998-11-27 [J. Chromatogr. A. 826 , 167-181, (1998)] |