Discovering thiamine transporters as targets of chloroquine using a novel functional genomics strategy.
Zhiwei Huang, Sankaranarayanan Srinivasan, Jianhuai Zhang, Kaifu Chen, Yongxiang Li, Wei Li, Florante A Quiocho, Xuewen Pan
文献索引:PLoS Genet. 8 , e1003083, (2012)
全文:HTML全文
摘要
Chloroquine (CQ) and other quinoline-containing antimalarials are important drugs with many therapeutic benefits as well as adverse effects. However, the molecular targets underlying most such effects are largely unknown. By taking a novel functional genomics strategy, which employs a unique combination of genome-wide drug-gene synthetic lethality (DGSL), gene-gene synthetic lethality (GGSL), and dosage suppression (DS) screens in the model organism Saccharomyces cerevisiae and is thus termed SL/DS for simplicity, we found that CQ inhibits the thiamine transporters Thi7, Nrt1, and Thi72 in yeast. We first discovered a thi3Δ mutant as hypersensitive to CQ using a genome-wide DGSL analysis. Using genome-wide GGSL and DS screens, we then found that a thi7Δ mutation confers severe growth defect in the thi3Δ mutant and that THI7 overexpression suppresses CQ-hypersensitivity of this mutant. We subsequently showed that CQ inhibits the functions of Thi7 and its homologues Nrt1 and Thi72. In particular, the transporter activity of wild-type Thi7 but not a CQ-resistant mutant (Thi7(T287N)) was completely inhibited by the drug. Similar effects were also observed with other quinoline-containing antimalarials. In addition, CQ completely inhibited a human thiamine transporter (SLC19A3) expressed in yeast and significantly inhibited thiamine uptake in cultured human cell lines. Therefore, inhibition of thiamine uptake is a conserved mechanism of action of CQ. This study also demonstrated SL/DS as a uniquely effective methodology for discovering drug targets.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
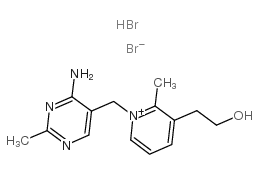 |
Pyrithiamine (hydrobromide)
CAS:534-64-5 |
C14H20Br2N4O |
|
Interactions between chronic ethanol consumption and thiamin...
2015-11-01 [Alcohol. Clin. Exp. Res. 39 , 2143-53, (2015)] |
|
Identification of the antiphagocytic trypacidin gene cluster...
2015-12-01 [Appl. Microbiol. Biotechnol. 99 , 10151-61, (2015)] |
|
Thiamine deficiency caused by thiamine antagonists triggers ...
2007-01-01 [Acta Biochim. Pol. 54(2) , 315-22, (2007)] |
|
Comparative characteristic of thiamine antagonists on apopto...
[Ukr. Biokhim. Zh. 80(5) , 76-84, (2008)] |
|
Identification of vitamin B1 metabolism as a tumor-specific ...
2015-03-20 [Oncotarget 6(8) , 5978-89, (2015)] |