| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
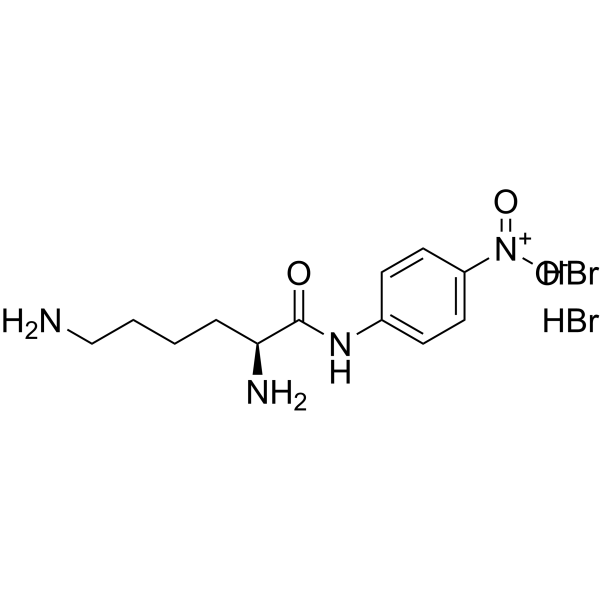 |
(2S)-2,6-二氨基-N-(4-硝基苯基)己酰胺二氢溴酸盐
CAS:40492-96-4 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
(2S)-2,6-二氨基-N-(4-硝基苯基)己酰胺二氢溴酸盐
CAS:40492-96-4 |