Proton NMR studies of the molecular basis for the anti-sickling activity of non-covalent antisickling compounds.
C Ho, I M Russu
文献索引:Prog. Clin. Biol. Res. 240 , 59-66, (1987)
全文:HTML全文
摘要
Two new techniques of nuclear magnetic resonance spectroscopy are proposed to identify and characterize the binding sites of antisickling compounds to Hb S: measurements of the longitudinal relaxation rates (T1(-1)) of the C2 protons of individual surface histidyl residues of the C2 protons of individual surface histidyl residues of Hb S and intermolecular transferred nuclear Overhauser effects from protons in the heme pockets of the Hb S molecule. Using these methods, we have investigated the binding sites of L-phenylalanine, L-tryptophan, L-valine, and p-bromobenzylalcohol to sickle hemoglobin. With the exception of L-valine, all of these molecules are known to inhibit the polymerization of deoxy Hb S. We have found that for the compounds with antisickling activity, there are at least two binding sites to the Hb S molecule, one at or near the heme pockets of the alpha and beta chains and the other one in the vicinity of the beta 6 mutation site.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
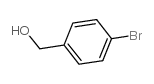 |
4-溴苄醇
CAS:873-75-6 |
C7H7BrO |
|
One-pot synthesis of ferrocenyl ketones containing biaryls a...
2013-09-06 [J. Org. Chem. 78(17) , 8730-8, (2013)] |
|
Active site specific cadmium(II)-substituted horse liver alc...
1985-12-03 [Biochemistry 24(25) , 7503-10, (1985)] |
|
Molecular basis for the anti-sickling activity of aromatic a...
1986-02-25 [Biochemistry 25(4) , 808-15, (1986)] |
|
Polyvinylpolypyrrolidone-supported hydrogen peroxide (PVP-H2...
[J. Iranian Chem. Soc. 8(4) , 1082-1090, (2011)] |
|
Protection of Hydroxyl Groups as a Trimethylsilyl Ether by 1...
[Chin. J. Catal. 32(3) , 595-598, (2011)] |