Rotational spectroscopy and molecular structure of the 1,1,2-trifluoroethylene-acetylene complex.
Helen O Leung, Mark D Marshall, Winn T Cashion, Vincent L Chen
文献索引:J. Chem. Phys. 128(6) , 064315, (2008)
全文:HTML全文
摘要
Guided by ab initio calculations, Fourier transform microwave rotational spectra in the 6-22 GHz region are obtained for the complex formed between 1,1,2-trifluoroethylene and acetylene, including the normal isotopomer, three of four singly substituted (13)C species obtained in natural abundance, and using commercially available isotopic varieties of acetylene, species containing HCCD and H(13)C(13)CH. Although the ab initio calculations suggest two possible low energy planar arrangements for the molecules in the complex, only a single, unique structure is obtained from a combined analysis of the rotational constants derived from the spectra and atomic positions determined using Kraitchman [Am. J. Phys. 21, 17 (1953)] substitution coordinates. This structure is similar to that obtained for the CF(2)CHF[Single Bond]HF complex [H. O. Leung and M. D. Marshall, J. Chem. Phys. 126, 114310 (2007)] in which both the primary and secondary interactions occur between the HCCH molecule and a F atom and a H atom bonded to the same carbon of CF(2)CHF. The 2.748(15) A hydrogen bond has acetylene as the donor and 1,1,2-trifluoroethylene as the acceptor and forms a 104.49(15) degrees C[Single Bond]Fcdots, three dots, centeredH angle. The 2.8694(9) A secondary interaction between the pi bond of acetylene and the H atom geminal to the acceptor F atom causes the hydrogen bond to deviate 69.24(67) degrees from linearity. This large deviation from linearity and the similarity of the two intermolecular bond lengths suggest that the two interactions are becoming comparable in importance.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
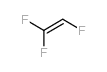 |
三氟乙烯
CAS:359-11-5 |
C2HF3 |
|
Analysis of peptide rotational diffusion by homonuclear NMR.
2002-04-15 [Biopolymers 63(5) , 335-42, (2002)] |
|
Effects of low-frequency ultrasound combined with microbubbl...
2013-01-01 [Can. Urol. Assoc. J. 7(11-12) , E681-6, (2013)] |
|
A 40-100 MHz B-scan ultrasound backscatter microscope for sk...
1995-01-01 [Ultrasound Med. Biol. 21(1) , 79-88, (1995)] |
|
Microporous small diameter PVDF-TrFE vascular grafts fabrica...
1992-01-01 [ASAIO J. 38(3) , M201-6, (1992)] |
|
Anionic collagen: polymer composites with improved dielectri...
1998-03-01 [Artif. Organs 22(3) , 203-9, (1998)] |