Crystal structure of NS-134 in complex with bovine cathepsin B: a two-headed epoxysuccinyl inhibitor extends along the entire active-site cleft.
Igor Stern, Norbert Schaschke, Luis Moroder, Dusan Turk
文献索引:Biochem. J. 381(Pt 2) , 511-7, (2004)
全文:HTML全文
摘要
The crystal structure of the inhibitor NS-134 in complex with bovine cathepsin B reveals that functional groups attached to both sides of the epoxysuccinyl reactive group bind to the part of active-site cleft as predicted. The -Leu-Pro-OH side binds to the primed binding sites interacting with the His110 and His111 residues with its C-terminal carboxy group, whereas the -Leu-Gly-Meu (-Leu-Gly-Gly-OMe) part (Meu, methoxycarbonylmethyl) binds along the non-primed binding sites. Comparison with the propeptide structures of cathepsins revealed that the binding of the latter part is least similar to the procathepsin B structure; this result, together with the two-residue shift in positioning of the Leu-Gly-Gly part, suggests that the propeptide structures of the cognate enzymes may not be the best starting point for the design of reverse binding inhibitors.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
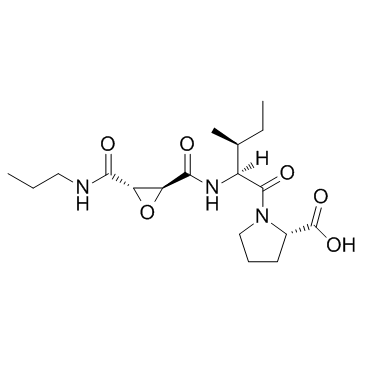 |
CA-074
CAS:134448-10-5 |
C18H29N3O6 |
|
Human cystatin C: a new biomarker of idiopathic pulmonary fi...
2014-06-01 [Proteomics. Clin. Appl. 8(5-6) , 447-53, (2014)] |
|
Redox-based inactivation of cysteine cathepsins by compounds...
2011-01-01 [PLoS ONE 6(11) , e27197, (2011)] |
|
Structural basis for development of cathepsin B-specific non...
2002-06-03 [Biochim. Biophys. Acta 1597(2) , 244-51, (2002)] |
|
Primate neurons show different vulnerability to transient is...
2002-09-01 [Acta Neuropathol. 104(3) , 267-72, (2002)] |
|
Role of the mitochondria in immune-mediated apoptotic death ...
2011-01-01 [PLoS ONE 6 , e20617, (2011)] |