Repellent activities of stereoisomers of p-menthane-3,8-diols against Anopheles gambiae (Diptera: Culicidae).
Stephen S Barasa, Isaiah O Ndiege, Wilber Lwande, Ahmed Hassanali
文献索引:J. Med. Entomol. 39(5) , 736-41, (2002)
全文:HTML全文
摘要
Four stereoisomers of p-menthane-3,8-diol, which make up the natural product obtained from Eucalyptus citriodora, were synthesized through stereoselective procedures. Repellency assays showed that all the four were equally active against Anopheles gambiae s.s. Racemic blends and the diastereoisomeric mixture of all the four isomers were also equally repellent. 1-alpha-terpeneol, with a single hydroxyl function at C-8 and unsaturation at C-8, and menthol, with a single hydroxyl function at C-3, were not repellent. The practical implication of these results is discussed.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
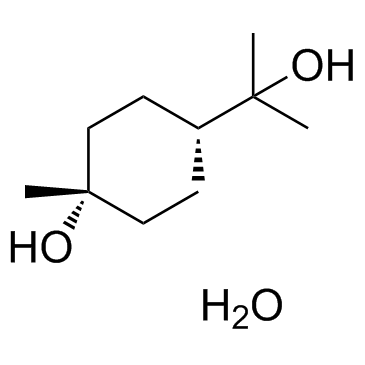 |
对-薄荷烷-1,8-二醇一水合物
CAS:2451-01-6 |
C10H22O3 |
|
A low-cost repellent for malaria vectors in the Americas: re...
2007-01-01 [Malaria Journal 6 , 101, (2007)] |
|
Percutaneous absorption of an insect repellent p-menthane-3,...
2009-01-01 [J. Toxicol. Environ. Health A 72(13) , 796-806, (2009)] |
|
Enhancement effect of p-menthane-3,8-diol on in vitro permea...
2004-07-01 [Drug Dev. Ind. Pharm. 30(6) , 673-7, (2004)] |
|
Repellency of oils of lemon eucalyptus, geranium, and lavend...
2006-07-01 [J. Med. Entomol. 43(4) , 731-6, (2006)] |
|
Repellency of MyggA Natural spray (para-menthane-3,8-diol) a...
2006-01-01 [Exp. Appl. Acarol. 40(3-4) , 271-7, (2006)] |