Mechanism of formation of reovirus mRNA 5'-terminal blocked and methylated sequence, m7GpppGmpC.
N Kobayashi, F Hariguchi, T Okamoto, Y Okada, T Hayashi, T Matsuno
文献索引:J. Biol. Chem. 251(16) , 5043-53, (1976)
全文:HTML全文
摘要
Blocked and methylated 5' termini of reovirus mRNA are formed by viral cores at an early stage of transcription. Cores incubated in a complete transcription reaction mixture for 30 s, or in a mixture lacking UTP and ATP for a longer time, synthesize the "cap" structure, m7GpppGmpC. The dinucleotide ppGpC functions as substrate for a core-associated guanylyltransferase and is converted to GpppGpC by addition of pG from GTP. For optimal conversion, both the diphosphate terminus and phosphodiester bond are required. pGpC is not a substrate, but pppGpC is utilized after removal of the gamma-phosphate by a core nucleotide phospohydrolase. Methyltransferases also present in cores transfer methyl groups sequentially from S-adenosylmethionine (AdoMet) to the N7-position of the 5'-terminal guanosine followed by the 2'-OH of the penultimate guanosine. GpppGpC is hydrolyzed by cores in the presence of pyrophosphate to ppGpC, the predominant 5'-terminal structure of reovirus mRNA made in the absence of S-adenosylmethionine. N7-methylation prevents pyrophosphorolysis of m7GpppGpC, which may explain the increased proportion of blocked, methylated 5' termini in viral mRNA synthesized in the presence of S-adenosylmethionine. On the basis of these findings, the following reaction series is proposed for the synthesis of reovirus mRNA caps. In the series, AdoHcy is the abbreviation for S-adenosylhomocysteine (see article)9
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
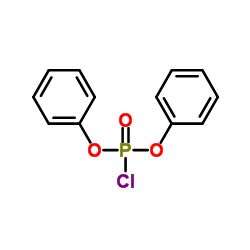 |
氯磷酸二苯酯
CAS:2524-64-3 |
C12H10ClO3P |
|
[Tetrahedron Lett. 34 , 2461, (1993)] |
|
[Carbohydr. Res. 223 , 169, (1992)] |
|
Synthesis of Disodium Phenyl Phosphate. Freeman HF and Colv...
[J. Am. Chem. Soc. 60(4) , 750-51, (1938)] |
|
219. Deoxy-sugars. Part XV. D-Galactose-3 and-6 phosphoric a...
[J. Chem. Soc. , 980-87, (1951)] |
|
Dialkyl (aryl) phosphoryl derivatives of alkylene dithiophos...
[Phosphorus. Sulfur. Silicon Relat. Elem. 179(9) , 1793-98, (2004)] |