DNA adducts of acrolein: site-specific synthesis of an oligodeoxynucleotide containing 6-hydroxy-5,6,7,8-tetrahydropyrimido[1,2-a]purin-10(3H)-one, an acrolein adduct of guanine.
Lubomir V Nechev, Ivan D Kozekov, Angela K Brock, Carmelo J Rizzo, Thomas M Harris
文献索引:Chem. Res. Toxicol. 15(5) , 607-13, (2002)
全文:HTML全文
摘要
3-(2-Deoxy-beta-D-erythro-pentofuranosyl)-6-hydroxy-5,6,7,8-tetrahydropyrimido[1,2-a]purin-10(3H)-one is formed in low yield by the reaction of acrolein with 2'-deoxyguanosine. The nucleoside and an oligodeoxynucleotide containing it have been synthesized. For preparation of the nucleoside 2'-deoxyguanosine was alkylated at the N1 position using 1-bromo-3-butene to give 1-(3-butenyl)-2'-deoxyguanosine. Oxidation with OsO(4) and N-methylmorpholine-N-oxide to give the 3,4-dihydroxybutyl adduct followed by oxidation with NaIO(4) gave the 1-(3-oxopropyl) adduct which cyclized spontaneously to yield the title compound as a rapidly epimerizing mixture of two diastereomers. Reduction of the nucleoside with NaBH(4) gave the unfunctionalized compound plus 1-(3-hydroxypropyl)-2'-deoxyguanosine showing that epimerization was occurring via both the imine and the 1-(3-oxopropyl) adduct. Reduction with NaCNBH(3) gave exclusively unfunctionalized 3-(2-deoxy-beta-D-erythro-pentofuranosyl)-5,6,7,8-tetrahydropyrimido[1,2-a]purin-10(3H)-one. The phosphoramidite reagent needed for preparation of oligonucleotides was prepared from 1-(3-butenyl)-2'-deoxyguanosine by glycolation after protection of the 3' and 5' hydroxyl groups as silyl derivatives. Acetylation of the vicinal hydroxyl groups and the exocyclic amino group followed by removal of silyl protection gave the protected nucleoside. Protection of the 5' hydroxyl group as the 4,4'-dimethoxytrityl ether followed by phosphitylation with 2-cyanoethyl-N,N,N',N'-tetraisopropylphosphorodiamidite gave the prosphoramidite reagent which was used to prepare a 12-mer oligodeoxynucleotide.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
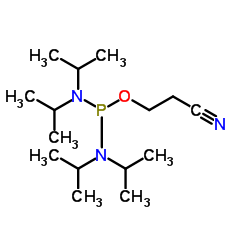 |
双(二异丙基氨基)(2-氰基乙氧基)膦
CAS:102691-36-1 |
C15H32N3OP |
|
2'-fluoro-4'-thioarabino-modified oligonucleotides: conforma...
2007-01-01 [Nucleic Acids Res. 35(5) , 1441-51, (2007)] |
|
Synthesis and fluorescence studies of multiple labeled oligo...
2004-01-01 [Bioconjug. Chem. 15 , 638-646, (2004)] |
|
Application of 2-cyanoethyl N,N,N',N'-tetraisopropylphosphor...
1986-09-25 [Nucleic Acids Res. 14(18) , 7391-403, (1986)] |
|
Synthesis of 1,2-diacyl-sn-glycerophosphatidylserine from eg...
1996-05-01 [Lipids 31(5) , 541-6, (1996)] |
|
LNA guanine and 2,6-diaminopurine. Synthesis, characterizati...
2004-05-01 [Bioorg. Med. Chem. 12 , 2385-2396, (2004)] |