Synthesis and stereoselective kappa-receptor binding of methylated analogues of GR-89.696.
C Röhr, S Soukara, B Wünsch
文献索引:Eur. J. Med. Chem. 36(2) , 211-4, (2001)
全文:HTML全文
摘要
Stereoselective synthesis of all four stereoisomers of methylated analogues 8 of the kappa-receptor agonist GR-89.696 is presented. Starting with orthogonally protected piperazine derivatives (R,R)-4 and (S,S)-4, the reaction sequence involves oxidation, reductive amination and modification of the piperazine nitrogen protective groups. The configuration of the stereocentre in alpha-position to the pyrrolidine moiety is determined by X-ray structure analysis of (R,S)-8. In receptor-binding studies with the radioligand U-69.593, the stereoisomer with (S)-configuration at both stereogenic centres (S,S)-8 displayed the highest kappa-receptor affinity with a K(i)-value of 0.67 nM.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
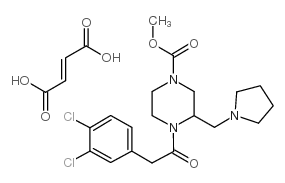 |
GR 89696 fumarate
CAS:126766-32-3 |
C23H29Cl2N3O7 |
|
[11C]GR103545: novel one-pot radiosynthesis with high specif...
2011-02-01 [Nucl. Med. Biol. 38(2) , 215-21, (2011)] |
|
GR89,696: a potent kappa-opioid agonist with subtype selecti...
2001-09-01 [J. Pharmacol. Exp. Ther. 298(3) , 1049-59, (2001)] |
|
[(11)C]-GR89696, a potent kappa opiate receptor radioligand;...
2002-01-01 [Nucl. Med. Biol. 29(1) , 47-53, (2002)] |
|
Sensory nerve-mediated relaxation of guinea-pig isolated pul...
1993-05-01 [Br. J. Pharmacol. 109(1) , 126-30, (1993)] |
|
Induction of bladder sphincter dyssynergia by kappa-2 opioid...
2004-01-01 [J. Urol. 171(1) , 472-7, (2004)] |