A double-blind, randomized comparative trial: flutrimazole 1% solution versus bifonazole 1% solution once daily in dermatomycoses.
del Palacio, S Cuétara, I Izquierdo, S Videla, J Delgadillo, E Boncompte, A Rodríguez Noriega
文献索引:Mycoses 38(9-10) , 395-403, (1995)
全文:HTML全文
摘要
In a double-blind, randomized study the efficacy and tolerance of flutrimazole 1% solution were compared with bifonazole 1% solution, applied once daily for 4 weeks, in 40 patients with culturally proven dermatophytosis or cutaneous candidosis. Forty patients with mycologically proven pityriasis versicolor were treated with once-daily application for 1 week. The four groups of patients and distribution of target lesions were similar, although in the flutrimazole group more patients had cutaneous candidosis (n = 8 versus n = 1). The distribution of the sum of clinical scores was also similar in both groups. At the end of therapy the proportion of patients with negative microscopy and culture was 85% in the flutrimazole group and 65% in the bifonazole group. There was a significant difference (P = 0.022) in terms of efficacy, since 80% of patients in the flutrimazole group versus 40% in the bifonazole group were judged to have received effective treatment. At the assessment 6 weeks after the end of therapy the percentages of flutrimazole- and bifonazole-treated patients with negative mycology were 75% and 65% respectively. There were two relapses (one in each group), which represents a 5% rate. Fifteen flutrimazole-treated patients (75%) compared with 12-bifonazole-treated patients (60%) had overall effective therapy. Two patients treated with bifonazole (10%) and one treated with flutrimazole (5%) had a premature termination due to adverse events attributable to the medication. On assessment 3 weeks after the end of treatment, the patients with pityriasis versicolor were all clinically and mycologically healed with negative fluorescence, including the patients who withdrew from the full course of treatment (one in each group). Nine weeks after the end of therapy all the patients remained cured, with no relapses. The overall incidence of adverse events (mild local reactions such as irritation, burning and itching) was one and seven cases for bifonazole and flutrimazole respectively. One patient in each group had to abandon treatment owing to severe intolerance.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
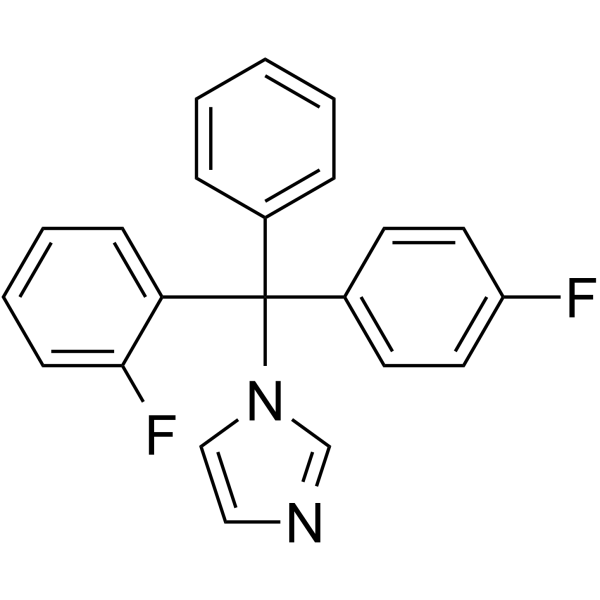 |
氟曲马唑
CAS:119006-77-8 |
C22H16F2N2 |
|
Double-blind randomized dose-finding study in acute vulvovag...
2000-10-01 [Mycoses 43(9-10) , 355-65, (2000)] |
|
Efficacy of flutrimazole 1% powder in the treatment of tinea...
2003-04-01 [Mycoses 46(3-4) , 126-31, (2003)] |
|
Study to compare the efficacy and safety of fluconazole crea...
2010-11-01 [Mycoses 53(6) , 522-9, (2010)] |
|
Topical anti-inflammatory properties of flutrimazole, a new ...
1996-01-01 [Inflamm. Res. 45(1) , 20-5, (1996)] |
|
Absorption and excretion of radioactivity after intravaginal...
1998-05-01 [Arzneimittelforschung 48(5) , 512-7, (1998)] |