The cysteine residues of recombinant human gastric lipase.
S Canaan, M Rivière, R Verger, L Dupuis
文献索引:Biochem. Biophys. Res. Commun. 257(3) , 851-4, (1999)
全文:HTML全文
摘要
Recombinant human gastric lipase (rHGL) and three of its cysteine mutants (cysteine 227, 236, and 244 substitued for threonine or serine) were expressed in the baculovirus/insect cell system and purified to homogeneity by performing a two-step procedure. Substituting Ser for Cys 227 and Cys 236 resulted in mutant lipases with a significantly lower level of activity (30% and 22%, respectively) on a short chain triglyceride (tribuyrin) substrate, while the mutation at position 244 only slightly reduced the activity. Using 4, 4'-dithiopyridine (4-PDS) as a sulfhydryl reagent on the above mutants, it was possible to clearly identify the single sulfhydryl residue at position 244 and consequently, the disulfide bridge at position 227-236. No potential disulfide bridges were formed during the protein folding between cysteines 227-244 or between cysteines 236-244, as thought to occur in the case of rabbit gastric lipase (RGL). The present results are consistent with the recently determined 3D-structure of rHGL.Copyright 1999 Academic Press.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
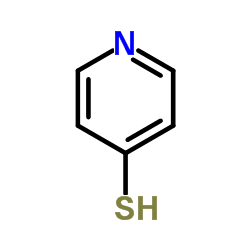 |
4-巯基吡啶
CAS:4556-23-4 |
C5H5NS |
|
Ab initio studies of some amino acid residue complexes with ...
1999-01-01 [Cancer Invest. 17(6) , 396-401, (1999)] |
|
Peptide-tailored assembling of Au nanorods.
2011-05-21 [Chem. Commun. (Camb.) 47(19) , 5482-4, (2011)] |
|
The time course of the interaction of sheep liver cytoplasmi...
1984-11-01 [Arch. Biochem. Biophys. 234(2) , 487-96, (1984)] |
|
In vitro-refolding of a single-chain Fv fragment in the pres...
2008-04-30 [J. Biotechnol. 134(3-4) , 218-21, (2008)] |
|
Synthesis of methyl 2- and 4-pyridyl disulfide from 2- and 4...
1985-05-01 [Anal. Biochem. 146(2) , 429-30, (1985)] |