[Preparation of derivatives of 3-(3,4-dimethoxyphenyl)propanic acid and a study of its biological activity].
L Novácek, O Nováková, L Polásek, J Danĕk
文献索引:Cesk. Farm. 39(3) , 109-12, (1990)
全文:HTML全文
摘要
From 3-(3,4-dimethoxyphenyl)propenic acid chloride and substituted amines and hydrazides, the appropriate amides and hydrazides (Table 1) were synthesized at 60-80 degrees C in the medium of benzene or toluene. The reaction of this chloride with benzaldehyde hydrazone at 70-80 degrees C yielded N,N'-bis[3-(3,4-dimethoxyphenyl)propenoyl]hydrazine (VIII). At ambient temperature the benzylidene hydrazide of 3-(3,4-dimethoxyphenyl)propenic acid (VII) and a small amount of compound VIII were isolated. In the reaction of 3-(3,4-dimethoxyphenyl)propenic acid chloride with benzylidene hydrazide (VII) at 70-80 degrees C, compound VIII was obtained (Scheme 1). Compounds I, VII, VIII and IX possessed higher indices of increase in fortnight tests of the first degree in the roosters of meat hybrides compared to the negative control, but the indices of conversion were unfavourable. The compounds did not reach the efficacy of the avoparcin standard. Derivatives II and VI possessed 67.5 and 63.5%, respectively, of anthelmintic activity of levamisol against Nippostrongylus brasiliensis. The prepared compounds were not antibacterially effective and they were not mutagenic in the tests following the method of Ames, either.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
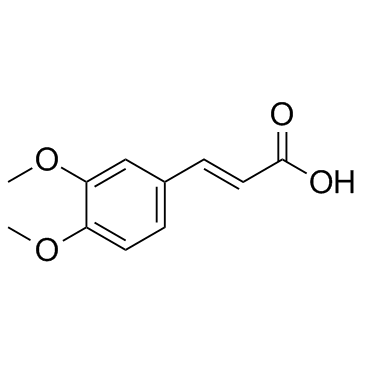 |
3,4-二甲氧基肉桂酸
CAS:2316-26-9 |
C11H12O4 |
|
Biosynthesis of podophyllotoxin in Linum album cell cultures...
2002-10-01 [Planta 215(6) , 1031-9, (2002)] |
|
[Chemical constituents of Veronicastrum sibiricum (L.) Penne...
1992-01-01 [Zhongguo Zhong Yao Za Zhi 17(1) , 35-6, 64, (1992)] |
|
[Pharmacological study on Veronicastrum sibiricum (L.) Penne...
1992-08-01 [Zhongguo Zhong Yao Za Zhi 17(8) , 493-6, inside backcover, (1992)] |
|
Absorption of dimethoxycinnamic acid derivatives in vitro an...
2012-09-01 [Mol. Nutr. Food. Res. 56(9) , 1413-23, (2012)] |
|
Determination of caffeine in coffee products by dynamic comp...
2007-10-01 [Electrophoresis 28(19) , 3570-4, (2007)] |