Synthesis of new 3-pyridinecarboxylates of potential vasodilation properties.
Adel S Girgis, Nawal Mishriky, Ahmad M Farag, Wafaa I El-Eraky, Hanaa Farag
文献索引:Eur. J. Med. Chem. 43(9) , 1818-27, (2008)
全文:HTML全文
摘要
2-(alicyclic-amino)-4,6-diaryl-3-pyridinecarboxylates 5a-d were prepared via aromatic nucleophilic substitution reaction of secondary amines (piperidine or morpholine) with 2-bromo-3-pyridinecarboxylate derivatives 3a,b. The latters were obtained through bromination of 3-aryl-4-benzoyl-2-cyanobutyrates 2a and 2b, which were obtained from the base promoted addition of ethyl cyanoacetate to 2-propen-1-ones 1a and 1b, with bromine in glacial acetic acid. Reaction of 3 with piperazine hexahydrate in 2:1 molar ratio afforded 1,4-bis[(ethyl 4,6-diaryl-3-pyridinecarboxylate)-2-yl]piperazines 6a,b. Reaction of 3 with anilines in refluxing pyridine unexpectedly gave 2-(aryl-amino)-3-pyridinecarboxylates 8a-g and 2-amino-3-pyridinecarboxylates 9a and 9b. Vasodilation activity screening for the synthesized pyridinecarboxylates using isolated thoracic aortic rings' standard method of rats shows considerable properties. Compounds 5b, 5c, 6b and 8g reveal remarkable vasodilation potency (IC50, concentrations necessary for 50% reduction of maximal norepinephrine hydrochloride induced contracture) 0.175, 0.146, 0.229 and 0.233 mM, respectively.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
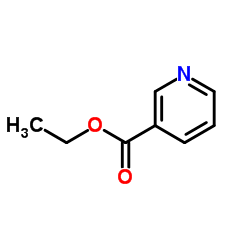 |
烟酸乙酯
CAS:614-18-6 |
C8H9NO2 |
|
In vitro metabolism and stability of the actinide chelating ...
2015-05-01 [J. Pharm. Sci. 104(5) , 1832-8, (2015)] |
|
Inhibitory effects of niacin and its analogues on induction ...
1987-09-15 [Biochem. Pharmacol. 36(18) , 3015-9, (1987)] |
|
Inhibitory effect of 3-carboethoxypyridine and 3-carbobutoxy...
2007-01-01 [Acta Pol. Pharm. 64(2) , 179-82, (2007)] |
|
Utility of a three-dimensional cultured human skin model as ...
2004-10-01 [Drug Metab. Pharmacokinet. 19(5) , 352-62, (2004)] |
|
Transdermal nicotine suppresses cutaneous inflammation.
1997-07-01 [Arch. Dermatol. 133(7) , 823-5, (1997)] |