Intramolecular hydroamination of aminoalkenes by calcium and magnesium complexes: a synthetic and mechanistic study.
Mark R Crimmin, Merle Arrowsmith, Anthony G M Barrett, Ian J Casely, Michael S Hill, Panayiotis A Procopiou
文献索引:J. Am. Chem. Soc. 28th ed., 131 , 9670-9685, (2009)
全文:HTML全文
摘要
The beta-diketiminate-stabilized calcium amide complex [{ArNC(Me)CHC(Me)NAr}Ca{N(SiMe(3))(2)}(THF)] (Ar = 2,6-diisopropylphenyl) and magnesium methyl complex [{ArNC(Me)CHC(Me)NAr}Mg(Me)(THF)] are reported as efficient precatalysts for hydroamination/cyclization of aminoalkenes. The reactions proceeded under mild conditions, allowing the synthesis of five-, six-, and seven-membered heterocyclic compounds. Qualitative assessment of these reactions revealed that the ease of catalytic turnover increases (i) for smaller ring sizes (5 > 6 > 7), (ii) substrates that benefit from favorable Thorpe-Ingold effects, and (iii) substrates that do not possess additional substitution on the alkene entity. Prochiral substrates may undergo diastereoselective hydroamination/cyclization depending upon the position of the existing stereocenter. Furthermore, a number of minor byproducts of these reactions, arising from competitive alkene isomerization reactions, were identified. A series of stoichiometric reactions between the precatalysts and primary amines provided an important model for catalyst initiation and suggested that these reactions are facile at room temperature, with the reaction of the calcium precatalyst with benzylamine proceeding with DeltaG(o)(298 K) = -2.7 kcal mol(-1). Both external amine/amide exchange and coordinated amine/amide exchange were observed in model complexes, and the data suggest that these processes occur via low-activation-energy pathways. As a result of the formation of potentially reactive byproducts such as hexamethyldisilazane, calcium-catalyst initiation is reversible, whereas for the magnesium precatalyst, this process is nonreversible. Further stoichiometric reactions of the two precatalysts with 1-amino-2,2-diphenyl-4-pentene demonstrated that the alkene insertion step proceeds via a highly reactive transient alkylmetal intermediate that readily reacts with N-H sigma bonds under catalytically relevant conditions. The results of deuterium-labeling studies are consistent with the formation of a single transient alkyl complex for both the magnesium and calcium precatalysts. Kinetic analysis of the nonreversible magnesium system revealed that the reaction rate depends directly upon catalyst concentration and inversely upon substrate concentration, suggesting that substrate-inhibited alkene insertion is rate-determining.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
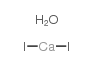 |
碘化钙水合物
CAS:71626-98-7 |
CaH2I2O |
|
Well-defined calcium initiators for lactide polymerization.
2004-10-18 [Inorg. Chem. 21th ed., 43 , 6717-6725, (2004)] |
|
Two Carbonylations of Methyl Iodide and Trimethylamine to Ac...
[Catalysts 5(4) , 1969-1982, (2015)] |
|
Compounds with one Saturated Heteroatom Bond-chlorine,bromin...
[Science of Synthesis: Houben-Weyl Methods of Molecular Transformations , (2006) 35] |
|
Jain, S. K.; et al.
[Indian J. Chem. A 38 , 778, (1999)] |
|
Novosad, S.S.; et al.
[Inorg. Mater. 8th ed., 44 , 900-904, (2008)] |