Ring oxidation of 6-nitrobenzo (a) pyrene by female mouse liver.
C Raha, D Williamson, M Hart-Anstey, T Maiefski, S J Stohs, E Bresnick
文献索引:Res. Commun. Chem. Pathol. Pharmacol. 58(1) , 63-74, (1987)
全文:HTML全文
摘要
6-Nitrobenzo (a) pyrene (6-NBaP) is an environmental contaminant. In bacterial mutagenesis assays, 6-NBaP requires rat liver S9 enzymes for its activity. Chemical characterization of metabolites of 6-NBaP produced by male rat liver microsomes showed them to be ring-hydroxylated (both mono- and dihydroxy) derivatives. Thus, metabolic activation of 6-NBaP may occur via ring oxidation. It has been shown by others that when injected intraperitoneally into newborn mice, 6-NBaP was carcinogenic to male, but not to female, mouse liver. The nitro-hydrocarbon was not carcinogenic to the lungs of either sex. We have examined the metabolism of 6-NBaP by the liver of a non-susceptible female mouse and observed the formation of ring-hydroxylated metabolites of 6-NBaP. In view of this observation, we suggest that ring hydroxylation alone may not be sufficient in explaining the carcinogenicity of 6-NBaP in male and not in female mice.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
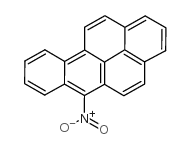 |
6-硝基苯并(A)芘
CAS:63041-90-7 |
C20H11NO2 |
|
Comparative lung tumorigenicity of parent and mononitro-poly...
1989-07-01 [Toxicol. Appl. Pharmacol. 99(3) , 555-63, (1989)] |
|
Metabolism of isomeric nitrobenzo[a]pyrenes leading to DNA a...
1997-05-12 [Mutat. Res. 376(1-2) , 43-51, (1997)] |
|
Structure and vibrational spectra of mononitrated benzo[a]py...
2006-01-12 [J. Phys. Chem. A 110(1) , 76-84, (2006)] |
|
Mutagenicity of the mononitrobenzo[a]pyrenes in Chinese hams...
1986-04-01 [Carcinogenesis 7(4) , 681-4, (1986)] |
|
Transforming activity of selected polycyclic aromatic hydroc...
1994-07-01 [Food Chem. Toxicol. 32(7) , 611-5, (1994)] |