Roles of formin nodes and myosin motor activity in Mid1p-dependent contractile-ring assembly during fission yeast cytokinesis.
Valerie C Coffman, Aaron H Nile, I-Ju Lee, Huayang Liu, Jian-Qiu Wu
文献索引:Mol. Biol. Cell 20 , 5195-210, (2009)
全文:HTML全文
摘要
Two prevailing models have emerged to explain the mechanism of contractile-ring assembly during cytokinesis in the fission yeast Schizosaccharomyces pombe: the spot/leading cable model and the search, capture, pull, and release (SCPR) model. We tested some of the basic assumptions of the two models. Monte Carlo simulations of the SCPR model require that the formin Cdc12p is present in >30 nodes from which actin filaments are nucleated and captured by myosin-II in neighboring nodes. The force produced by myosin motors pulls the nodes together to form a compact contractile ring. Live microscopy of cells expressing Cdc12p fluorescent fusion proteins shows for the first time that Cdc12p localizes to a broad band of 30-50 dynamic nodes, where actin filaments are nucleated in random directions. The proposed progenitor spot, essential for the spot/leading cable model, usually disappears without nucleating actin filaments. alpha-Actinin ain1 deletion cells form a normal contractile ring through nodes in the absence of the spot. Myosin motor activity is required to condense the nodes into a contractile ring, based on slower or absent node condensation in myo2-E1 and UCS rng3-65 mutants. Taken together, these data provide strong support for the SCPR model of contractile-ring formation in cytokinesis.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
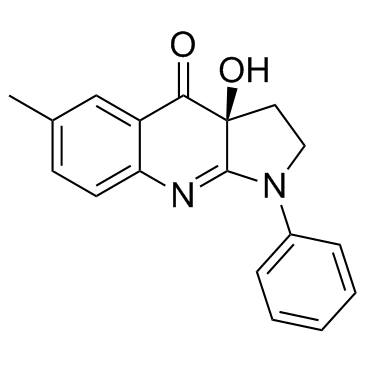 |
(-)-II型肌球蛋白
CAS:856925-71-8 |
C18H16N2O2 |
|
Stretch-stimulated glucose transport in skeletal muscle is r...
2015-02-01 [J. Physiol. 593(3) , 645-56, (2015)] |
|
Formation of contractile networks and fibers in the medial c...
2015-01-01 [Cytoskeleton (Hoboken.) 72(1) , 29-46, (2015)] |
|
Intramolecular loop/tail interactions are essential for conn...
2010-11-01 [FASEB J. 24 , 4378-95, (2010)] |
|
Glucocorticoid receptor-mediated expression of caldesmon reg...
2008-11-07 [J. Biol. Chem. , 31183-96, (2008)] |
|
Distinct cytoskeleton populations and extensive crosstalk co...
2011-04-01 [Development 138 , 1631-41, (2011)] |