Auxin binding proteins from maize coleoptiles: purification and molecular characterization.
K Palme, J Feldwisch, T Hesse, G Bauw, M Puype, J Vandekerchkhove, J Schell
文献索引:Symp. Soc. Exp. Biol. 44 , 299-313, (1990)
全文:HTML全文
摘要
To understand precisely the mechanisms by which hormones like auxins regulate plant differentiation and development, it is essential to isolate putative hormone receptors. We have purified the major auxin binding protein from maize coleoptiles to homogeneity. The protein has an apparent molecular weight of 22,000 Da and binds 1-naphthylacetic acid with a KD of 2.4 x 10(-7) M. Protein sequence analysis allowed the construction of oligonucleotide probes to isolate a corresponding cDNA coding for this protein. The open reading frame of this cDNA predicts a protein of 201 amino acids and 21,990 Da in size. The amino acid sequence includes a cleavable N-terminal signal sequence and a C-terminal signal element consisting of the amino acids Lys Asp Glu Leu known to be responsible for preventing secretion of proteins from the lumen of the endoplasmic reticulum.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
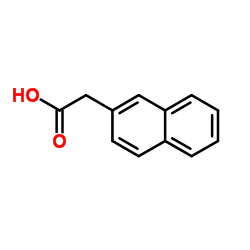 |
2-萘乙酸
CAS:581-96-4 |
C12H10O2 |
|
D-amino acids modulate the cellular response of enzymatic-in...
2014-10-13 [Biomacromolecules 15(10) , 3559-68, (2014)] |
|
Differentiation of esterases reacting with organophosphorus ...
1993-06-01 [Chem. Biol. Interact. 87(1-3) , 77-83, (1993)] |
|
Ceramide participates in lysosome-mediated cell death induce...
2015-06-01 [Leuk. Lymphoma 56 , 1863-8, (2015)] |
|
The hydrolytic activity of esterases in the yeast Saccharomy...
2011-11-01 [Cell Biol. Int. 35(11) , 1111-9, (2011)] |
|
Substrate specificity and enantioselectivity of arylcarboxyl...
1988-01-01 [Drug Metab. Dispos. 16(4) , 627-34, (1988)] |