Stabilization of Torpedo californica acetylcholinesterase by reversible inhibitors.
Lev Weiner, Valery L Shnyrov, Leonid Konstantinovskii, Esther Roth, Yacov Ashani, Israel Silman
文献索引:Biochemistry 48(3) , 563-74, (2009)
全文:HTML全文
摘要
The dimeric form of Torpedo californica acetylcholinesterase provides a valuable experimental system for studying transitions between native, partially unfolded, and unfolded states since long-lived partially unfolded states can be generated by chemical modification of a nonconserved buried cysteine residue, Cys 231, by denaturing agents, by oxidative stress, and by thermal inactivation. Elucidation of the 3D structures of complexes of Torpedo californica acetylcholinesterase with a repertoire of reversible inhibitors permits their classification into three categories: (a) active-site directed inhibitors, which interact with the catalytic anionic subsite, at the bottom of the active-site gorge, such as edrophonium and tacrine; (b) peripheral anionic site inhibitors, which interact with a site at the entrance to the gorge, such as propidium and d-tubocurarine; and (c) elongated gorge-spanning inhibitors, which bridge the two sites, such as BW284c51 and decamethonium. The effects of these three categories of reversible inhibitors on the stability of Torpedo californica acetylcholinesterase were investigated using spectroscopic techniques and differential scanning calorimetry. Thermodynamic parameters obtained calorimetrically permitted quantitative comparison of the effects of the inhibitors on the enzyme's thermal stability. Peripheral site inhibitors had a relatively small effect, while gorge-spanning ligands and those binding at the catalytic anionic site, had a much larger stabilizing effect. The strongest effect was, however, observed with the polypeptide toxin, fasciculin II (FasII), even though, in terms of its binding site, it belongs to the category of peripheral site ligands. The stabilizing effect of the ligands binding at the anionic subsite of the active site, like that of the gorge-spanning ligands, may be ascribed to their capacity to stabilize the interaction between the two subdomains of the enzyme. The effect of fasciculin II may be ascribed to the large surface area of interaction (>2000 A(2)) between the two proteins. Stabilization of Torpedo californica acetylcholinesterase by both divalent cations and chemical chaperones was earlier shown to be due to a shift in equilibrium between the native state and a partially unfolded state ( Millard et al. ( 2003 ) Protein Sci. 12 , 2337 - 2347 ). The low molecular weight inhibitors used in the present study may act similarly and can thus be considered as pharmacological chaperones for stabilizing the fully folded native form of the enzyme.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
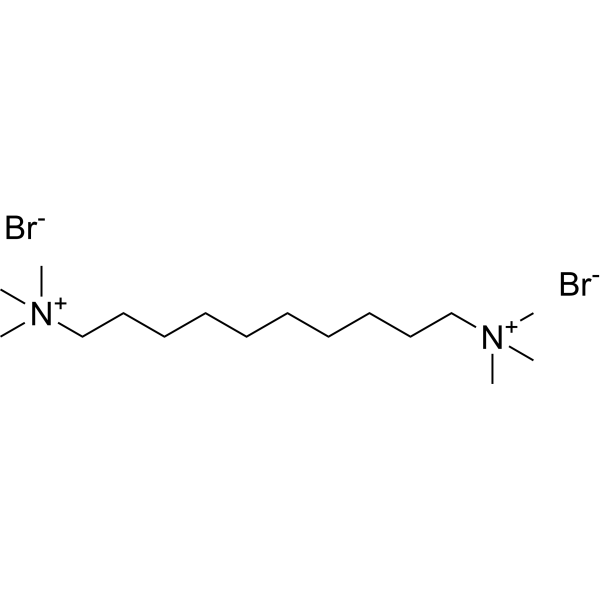 |
溴化十烃季胺
CAS:541-22-0 |
C16H38Br2N2 | |
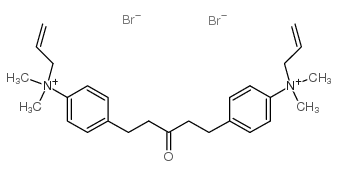 |
1,5-双(4-烯丙基二甲基氨苯基)戊-3-酮二溴
CAS:402-40-4 |
C27H38Br2N2O |
|
Chemical genetics reveals a complex functional ground state ...
2007-05-01 [Nat. Chem. Biol. 3(5) , 268-273, (2007)] |
|
Genetic mapping of targets mediating differential chemical p...
2009-10-01 [Nat. Chem. Biol. 5 , 765-71, (2009)] |
|
Development of a phospholipidosis database and predictive qu...
2008-01-01 [Toxicol. Mech. Methods 18 , 217-27, (2008)] |
|
Dequalinium-induced protofibril formation of alpha-synuclein...
2006-02-10 [J. Biol. Chem. 281(6) , 3463-72, (2006)] |
|
Long-lasting activation of rhythmic neuronal activity by a n...
2004-01-01 [J. Neurophysiol. 91(1) , 78-91, (2004)] |