Highly selective Diels-Alder reactions of directly connected enyne dienophiles.
Mingji Dai, David Sarlah, Maolin Yu, Samuel J Danishefsky, Gavin O Jones, K N Houk
文献索引:J. Am. Chem. Soc. 129 , 645, (2007)
全文:HTML全文
摘要
The paper describes the course of cycloadditions of Diels-Alder dienophiles containing linked enyne sites, each substituted with activating groups. Consistently, it was found that in the enyne cases the Diels-Alder reaction occurred specifically at the acetylenic center. Furthermore, it was found that the regiochemical sense of the cycloaddition was apparently determined by the remote activating group bound to the olefinic site. This remote acrylyl group totally dominated the course of the cycloaddition, relative to the activating group bound directly on the acetylene site. Explanations for these findings at the computational level are provided. The computations also rationalize the strong preference for cycloaddition to occur at the acetylene linkage and encompass the otherwise surprising regiochemical dominance by the remote ester on the olefinic site. The high selectivities available through such reactions provide important new opportunities in the synthesis of orsenillate type substructures that are found in a variety of natural products of contemporary interest.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
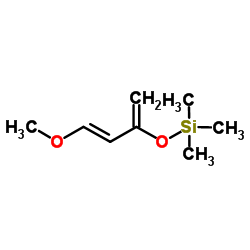 |
反-1-甲氧基-3-(三甲基硅氧基)-1,3-丁二烯
CAS:54125-02-9 |
C8H16O2Si |
|
Flexible protocol for the chemo- and regioselective building...
2008-05-15 [Org. Lett. 10(10) , 1983-6, (2008)] |
|
Cross-Diels-Alder reactions of 6-oxo-1-sulfonyl-1,6-dihydrop...
2007-03-30 [J. Org. Chem. 72 , 2364, (2007)] |
|
Sulfogriseofulvin derivatives. synthesis by [4 + 2]cycloaddi...
1996-07-01 [Arch. Pharm. (Weinheim) 329(7) , 361-70, (1996)] |
|
[Tetrahedron 49 , 1749, (1993)] |
|
[J. Org. Chem. 57 , 3605, (1992)] |