An evaluation of patient and physician satisfaction with controlled-release oxybutynin 15 mg as a one-step daily dose in elderly and non-elderly patients with overactive bladder: results of the STOP study.
Lorne E Aaron, Thomas J Morris, Paulette Jahshan, Joseph L Reiz
文献索引:Curr. Med. Res. Opin. 28(8) , 1369-79, (2012)
全文:HTML全文
摘要
Evaluate patient and physician satisfaction with a novel formulation of a once-daily controlled-release (CR) oxybutynin (Uromax*) 15-mg tablet as both the initial and maintenance dose in elderly and non-elderly patients with overactive bladder (OAB).Patients not on anticholinergic treatment for OAB and experiencing urinary incontinence (≥1 episode/week) and micturition frequency (≥8 episodes/day) or urgency (≥1 episode/week) were enrolled in this 4-week, open-label study. Satisfaction, efficacy, mental status and adverse events were evaluated by urologists, gynecologists, urogynecologists and family practitioners. The analyses compared the outcomes in patients <65 and ≥65 years.ISRCTN 19242032.A total of 240 patients enrolled; 111 (46%) were ≥65 years of age. Completion rate was 76.0% (<65) and 62.2% (≥65) (p = 0.0204). Medication was rated as tolerable by 75.2% of patients <65 and 58.6% of patients ≥65 (p = 0.0099). Based on overall satisfaction scores 64.2% (patient scores) and 57.1% (physician scores) of patients were considered 'successfully treated' (p = 0.0001 & p = 0.0451). There was a significant reduction in incontinence (64.3%; p = 0.0001), nocturia (38.6%; p = 0.0001) and night-time incontinence (39.7%; p = 0.0436) with no difference between age groups. Total continence was achieved by 29.8% and 47.5% of patients <65 and ≥65, respectively (p = 0.0077). No patients clinically experienced confusion or delirium and only six patients ≥65 had a decrease in MMSE score of ≥3 units, which was not statistically different from patients <65 (p = 0.3112). Dry mouth was the most common adverse event reported by 24.8% of patients <65 and 36.0% of patients ≥65 (p = 0.0584). Limitations of the study include a fixed dosing, no control group and 4-week trial.Patients and physicians were satisfied with CR oxybutynin 15 mg once-daily. Patients tolerated the CR oxybutynin 15 mg as both the initial and maintenance dose and provided significant reductions in incontinence, nocturia and night-time incontinence without a significant change in cognitive status. Total continence rates were significantly superior in patients ≥65 and there was no difference in dry mouth, cognitive status or efficacy in patients <65 and ≥65.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
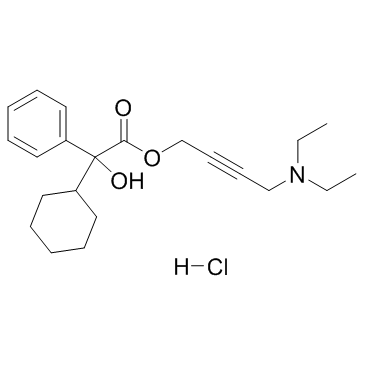 |
盐酸奥昔布宁
CAS:1508-65-2 |
C22H32ClNO3 |
|
Developing structure-activity relationships for the predicti...
2010-07-19 [Chem. Res. Toxicol. 23 , 1215-22, (2010)] |
|
A predictive ligand-based Bayesian model for human drug-indu...
2010-12-01 [Drug Metab. Dispos. 38 , 2302-8, (2010)] |
|
Chemical genetics reveals a complex functional ground state ...
2007-05-01 [Nat. Chem. Biol. 3(5) , 268-273, (2007)] |
|
Genetic mapping of targets mediating differential chemical p...
2009-10-01 [Nat. Chem. Biol. 5 , 765-71, (2009)] |
|
Investigating detrusor muscle concentrations of oxybutynin a...
2015-07-01 [J. Pharm. Sci. 104 , 2233-40, (2015)] |