| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
己二氯化膦
CAS:928-64-3 |
|
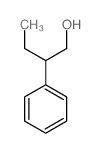 |
DL-β-乙基苯乙基乙醇
CAS:2035-94-1 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
己二氯化膦
CAS:928-64-3 |
|
 |
DL-β-乙基苯乙基乙醇
CAS:2035-94-1 |