Kinetic study of the gas-phase reactions of OH and NO3 radicals and O3 with selected vinyl ethers.
Shouming Zhou, Ian Barnes, Tong Zhu, Iustinian Bejan, Thorsten Benter
文献索引:J. Phys. Chem. A 110(23) , 7386-92, (2006)
全文:HTML全文
摘要
Kinetic studies on the gas-phase reactions of OH and NO3 radicals and ozone with ethyl vinyl ether (EVE), propyl vinyl ether (PVE) and butyl vinyl ether (BVE) have been performed in a 405 L borosilicate glass chamber at 298 +/- 3 K in synthetic air using in situ FTIR spectroscopy to monitor the reactants. Using a relative kinetic method rate coefficients (in units of cm3 molecule(-1) s(-1)) of (7.79 +/- 1.71) x 10(-11), (9.73 +/- 1.94) x 10(-11) and (1.13 +/- 0.31) x 10(-10) have been obtained for the reaction of OH with EVE, PVE and BVE, respectively, (1.40 +/- 0.35) x 10(-12), (1.85 +/- 0.53) x 10(-12) and (2.10 +/- 0.54) x 10(-12) for the reaction of NO3 with EVE, PVE and BVE, respectively, and (2.06 +/- 0.42) x 10(-16), (2.34 +/- 0.48) x 10(-16) and (2.59 +/- 0.52) x 10(-16) for the ozonolysis of EVE, PVE and BVE, respectively. Tropospheric lifetimes of EVE, PVE and BVE with respect to the reactions with reactive tropospheric species (OH, NO3 and O3) have been estimated for typical OH and NO3 radical and ozone concentrations.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
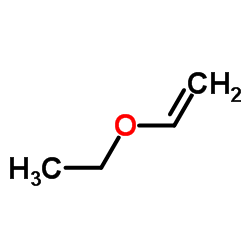 |
乙烯基乙醚
CAS:109-92-2 |
C4H8O |
|
Unraveling the interplay of backbone rigidity and electron r...
2014-09-03 [J. Am. Chem. Soc. 136(35) , 12479-88, (2014)] |
|
Well-defined diblock brush polymer-drug conjugates for susta...
2015-07-01 [Biomater. Sci. 3 , 1078-84, (2015)] |
|
Influence of Alkoxy Groups on the Photoinduced Dynamics of O...
2015-11-12 [J. Phys. Chem. A 119 , 11105-12, (2015)] |
|
Continuous-flow synthesis of monoarylated acetaldehydes usin...
2012-08-01 [J. Am. Chem. Soc. 134(30) , 12466-9, (2012)] |
|
Au(I)-catalyzed efficient synthesis of functionalized bicycl...
2008-06-04 [J. Am. Chem. Soc. 130(22) , 6944-5, (2008)] |