| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
聚合物负载 3,5-二甲基吡唑-1-甲脒盐酸盐
CAS:40027-64-3 |
|
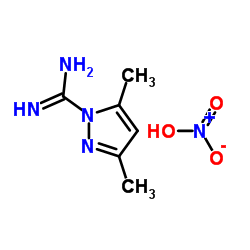 |
3,5-二甲基吡唑-1-硝酸咪
CAS:38184-47-3 |