| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
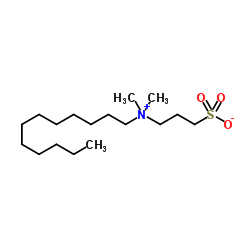 |
十二烷基二甲基(3-磺丙基)氢氧化铵内盐
CAS:14933-08-5 |
|
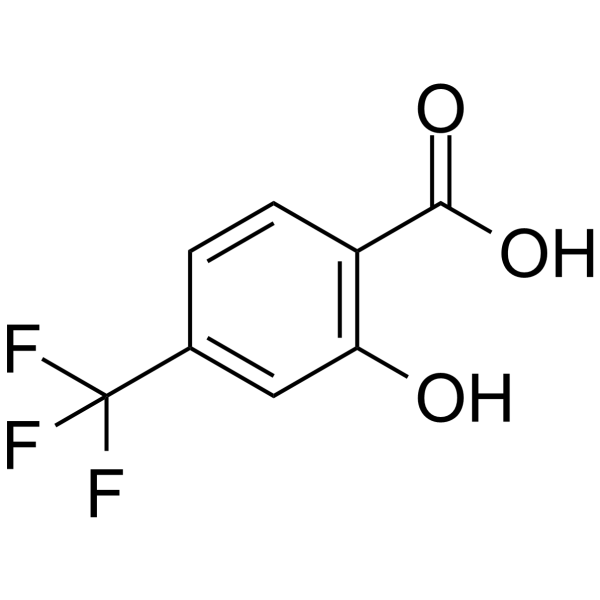 |
对三氟甲基水杨酸
CAS:328-90-5 |