Carryover effect on next-day sleepiness and psychomotor performance of nighttime administered antihistaminic drugs: a randomized controlled trial.
Yasuko Katayose, Sayaka Aritake, Shingo Kitamura, Minori Enomoto, Akiko Hida, Kiyohisa Takahashi, Kazuo Mishima
文献索引:Hum. Psychopharmacol. Clin. Exp. 27(4) , 428-36, (2012)
全文:HTML全文
摘要
Antihistamines with strong sedative-hypnotic properties are frequently prescribed for insomnia secondary to allergy, but the potential risks of such administration have not been fully elucidated.This randomized, double-blind, placebo-controlled crossover study was conducted to evaluate next-day sleepiness and psychomotor performance following the administration of antihistamines. Twenty-two healthy male participants participated in four drug administration sessions with more than a 1-week interval between the sessions. Either zolpidem 10 mg, or diphenhydramine 50 mg, or ketotifen 1 mg, or a placebo was administered before sleep, and polysomnography was conducted to evaluate sleep. In the morning and afternoon of the day after administration, the participants were evaluated for subjective sleepiness, objective sleepiness, and psychomotor performance.The antihistamines with high blood-brain barrier-crossing efficiency were significantly associated with sleepiness and psychomotor performance decline the next day. Ketotifen showed the strongest carryover effect, followed by diphenhydramine. Compared with the placebo, no significant carryover effect was observed with zolpidem.The results suggest that the risk-benefit balance should be considered in the ready use of antihistamines that easily cross the blood-brain barrier for alleviating secondary insomnia associated with allergies.Copyright © 2012 John Wiley & Sons, Ltd.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
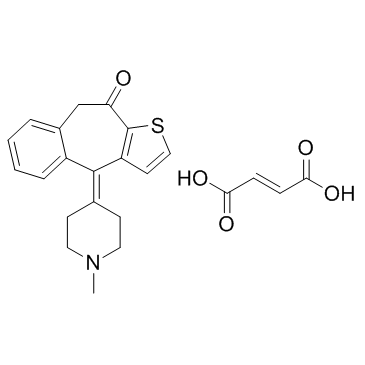 |
富马酸酮替芬
CAS:34580-14-8 |
C23H23NO5S |
|
Development and validation of an ultra-performance liquid ch...
2015-03-01 [Biomed. Chromatogr. 29(3) , 465-74, (2015)] |
|
[Mast cell activation disease: a concise practical guide for...
2014-07-01 [Dtsch. Med. Wochenschr. 139(30) , 1523-34; quiz 1535-8, (2014)] |
|
The effect of antihistamines on seizures induced by increasi...
2012-01-01 [Biol. Pharm. Bull. 35(5) , 693-7, (2012)] |
|
Novel delivery approach for ketotifen fumarate: dissofilms f...
2014-08-01 [Pharm. Dev. Technol. 19(5) , 521-30, (2014)] |
|
Eosinophilic ascites resolution with ketotifen.
2011-10-01 [Mayo Clin. Proc. 86(10) , 1027, (2011)] |